среда, 30 июня 2010 г.
вторник, 29 июня 2010 г.
Ларазидон: сравнение эффективности доз
Table 1. Comparison of Symptom Reduction
| Intervention | PANSS Positive Score Change | PANSS Negative Score Change |
| Placebo | -5.4 | -3.6 |
| Lurasidone 40 mg | -7.7 | -6.0 |
| Lurasidone 120 mg | -7.7 | -5.2 |
| Olanzapine | -9.3 | -6.2 |
Lurasidone, a novel antipsychotic agent submitted for review to the Food and Drug Administration, with high binding affinities for D2, 5-HT-7, 5-HT-2A, and 5-HT-1A receptors, and is being studied as an antipsychotic with reduced likelihood of causing weight gain, central nervous system depression, and orthostatic hypotension. The multi-center, phase 3 Program to Evaluate Antipsychotic Response to Lurasidone (PEARL 2) trial was conducted to evaluate the efficacy of 2 doses of lurasidone in the treatment of individuals experiencing an acute exacerbation of schizophrenia.
Lurasidone: A Novel, Weight-Neutral Antipsychotic Agent
Оланзапина памоат
In this well-designed study, depot olanzapine provided clinically significant prevention of psychotic relapse in outpatients treated with 3 dosing regimes. The highest dose was slightly more effective than the lowest dose but had a greater side effect burden. Injections of 405 mg every 4 weeks, more convenient and possibly safer than injections every 2 weeks, were effective and well tolerated. Because of the risk for postinjection sedation and delirium in approximately 7 injections in 10,000, a 3-hour postinjection observation period is required on the warning label approved by the FDA in December 2009. Given this and the risk for metabolic adverse effects, use of long-acting OP requires careful patient selection.
Recent Research in Antipsychotic Therapy
понедельник, 28 июня 2010 г.
Ламотриджин и ЭСТ
OBJECTIVE:: To evaluate the effect of lamotrigine (LMT) on electroconvulsive therapy (ECT)-induced seizures. METHODS:: Charts of all patients receiving LMT while undergoing an ECT course from July 2001 through May 2009 were reviewed. Apart from demographic variables, data collection consisted of diagnosis, indication for ECT, index or continuation ECT, electrode placement, stimulus dose, motor and electroencephalographic seizure duration, LMT dose, and number of restimulations. The stimulus dose and the seizure duration of ECT treatments with concurrent LMT (>/=200 mg/d) were compared with the stimulus dose and seizure duration of ECT treatments without concurrent LMT. RESULTS:: Lamotrigine was used by 19 patients (16 women, 3 men) during 289 treatment sessions. Eleven patients had ECT treatments with and without LMT, of which 8 were at a dosage of 200 mg/d or higher. Analyses did not reveal a significant difference in seizure duration and stimulus dose. Missed seizures, however, occurred more frequently during ECT treatments with concurrent LMT. CONCLUSIONS:: In all patients, seizures of adequate duration could be elicited. The combination was well tolerated. Therapeutic doses of LMT do not seem to have a clinically significant influence on the length of ECT-induced seizures nor on the stimulus dose.
Concurrent Use of Lamotrigine and Electroconvulsive Therapy.
Concurrent use of lamotrigine with ECT in bipolar depression seems safe, did not interfere with routine ECT practice, and allowed for transition to maintenance pharmacotherapy.
Combined Use of Lamotrigine and Electroconvulsive Therapy in Bipolar Depression: A Case Series
OBJECTIVES: To review the literature on the concurrent use of electroconvulsive therapy (ECT) and anticonvulsant drugs (AC) and to provide recommendations to guide clinical practice. METHODS: A MEDLINE search (1985-2006) was performed, using the terms "electroconvulsive therapy," "anticonvulsants," "epilepsy," "carbamazepine," "gabapentin," "lamotrigine," "topiramate," and "valproate," supplemented by manual searches of guidelines and textbooks on ECT. RESULTS: To date, no prospective, randomized and controlled trials examining outcome and safety of the AC-ECT combination have been published. Existing data are from case reports on the use of ECT for psychiatric conditions that are simultaneously treated with AC, and from case reports of patients treated with ECT and AC for epilepsy or for psychiatric conditions with comorbid epilepsy. Apart from an occasional difficulty in eliciting seizures, no severe adverse effects or complications are reported. CONCLUSIONS: The literature that is currently available indicates that ECT can be safely and effectively administered to patients treated with various AC. There is, however, no evidence to combine the 2 treatment modalities to augment therapeutic efficacy.
Anticonvulsants during electroconvulsive therapy: review and recommendations.
четверг, 24 июня 2010 г.
Временные обрывы маниакального состояния вестибулярной стимуляцией
Caloric vestibular stimulation is a common clinical procedure, routinely employed during testing of vestibulocochlear nerve function. The procedure involves stimulation of vestibular afferents by the application of cooled water to the tympanic membrane. Vestibular afferents are distributed widely to areas of the diencephalon and cortex, including areas believed to be involved in the regulation of mood. In accordance with these observations, imaging studies have shown widespread though largely contralateral hemispheric activation following the procedure.
Vestibular stimulation in mania: acase report
среда, 23 июня 2010 г.
Психозы гиперчувствительности, психозы "отдачи", поздние психозы
* Антиаритмические препараты могут провоцировать аритмии, антибиотики могут способствовоать развитию новых видов инфекций, а антипсихотические препараты могут вызывать психоз.
* Как мы можем отличить поздний психоз от шизофрении?
* Не является ли иногда резистентная шизофрения на самом деле поздним психозом?
* Снижает ли применение некоторых новых антипсихотиков вероятность развития позднего психоза?
АНТИПСИХОТИЧЕСКИЙ ПСИХОЗ
вторник, 22 июня 2010 г.
понедельник, 21 июня 2010 г.
Скополамин и депрессия
An intravenous infusion of scopolamine induced significant depression improvement in three to five days. The effect persisted for at least two weeks after three doses.
The hunt for a fast-acting antidepressant moved a step forward with a report of the efficacy of scopolamine, a drug commonly used to treat vertigo, in a clinical trial.
A muscarinic cholinergic receptor antagonist, scopolamine is commonly available in a type of transdermal patch indicated for treating motion sickness. The patch delivers up to one milligram of scopolamine over three days.
Vertigo Drug Shows Promise as Depression Treatment
пятница, 18 июня 2010 г.
Эквиваленты доз флуфеназина таблетированного и пролонга
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Fluphenazine decanoate injection may be given intramuscularly or subcutaneously. A dry syringe and needle of at least 21 gauge should be used. Use of a wet needle or syringe may cause the solution to become cloudy.
To begin therapy with fluphenazine decanoate the following regimens are suggested:
For most patients, a dose of 12.5 to 25 mg (0.5 to 1 mL) may be given to initiate therapy. The onset of action generally appears between 24 and 72 hours after injection and the effects of the drug on psychotic symptoms becomes significant within 48 to 96 hours. Subsequent injections and the dosage interval are determined in accordance with the patient’s response. When administered as maintenance therapy, a single injection may be effective in controlling schizophrenic symptoms up to four weeks or longer. The response to a single dose has been found to last as long as six weeks in a few patients on maintenance therapy.
It may be advisable that patients who have no history of taking phenothiazines should be treated initially with a shorter-acting form of fluphenazine before administering the decanoate to determine the patient’s response to fluphenazine and to establish appropriate dosage. For psychotic patients who have been stabilized on a fixed daily dosage of fluphenazine hydrochloride tablets, fluphenazine hydrochloride elixir, or fluphenazine hydrochloride oral solution, conversion of therapy from these short-acting oral forms to the long-acting injectable fluphenazine decanoate may be indicated.
Appropriate dosage of fluphenazine decanoate injection should be individualized for each patient and responses carefully monitored. No precise formula can be given to convert to use of fluphenazine decanoate; however, a controlled multicentered
study1, in patients receiving oral doses from 5 to 60 mg fluphenazine hydrochloride daily, showed that 20 mg fluphenazine hydrochloride daily was equivalent to 25 mg (1 mL) fluphenazine decanoate every three weeks. This represents an approximate conversion ratio of 0.5 mL (12.5 mg) of decanoate every three weeks for every 10 mg of fluphenazine hydrochloride daily.
Once conversion to fluphenazine decanoate is made, careful clinical monitoring of the patient and appropriate dosage adjustment should be made at the time of each injection.
Severely agitated patients may be treated initially with a rapid-acting phenothiazine compound such as fluphenazine hydrochloride injection (see package insert accompanying that product for complete information). When acute symptoms have subsided, 25 mg (1 mL) of fluphenazine decanoate may be administered; subsequent dosage is adjusted as necessary.
“Poor risk” patients (those with known hypersensitivity to phenothiazines, or with disorders that predispose to undue reactions): Therapy may be initiated cautiously with oral or parenteral fluphenazine hydrochloride (see package inserts accompanying these products for complete information). When the pharmacologic effects and an appropriate dosage are apparent, an equivalent dose of fluphenazine decanoate may be administered. Subsequent dosage adjustments are made in accordance with the response of the patient.
The optimal amount of the drug and the frequency of administration must be determined for each patient, since dosage requirements have been found to vary with clinical circumstances as well as with individual response to the drug.
Dosage should not exceed 100 mg. If doses greater than 50 mg are deemed necessary, the next dose and succeeding
doses should be increased cautiously in increments of 12.5 mg.
FLUPHENAZINE DECANOATE INJECTION, USP
вторник, 15 июня 2010 г.
Селективность антипсихотиков in vitro и ex vivo
In a recent human [11C]-(+)-PHNO positron emission tomography study, olanzapine, clozapine, and risperidone occupied D2 receptors in striatum (STR), but, despite their similar in vitro D2 and D3 affinities, failed to occupy D3 receptors in globus pallidus. This study had two aims: (1) to characterize the regional D2/D3 pharmacology of in vitro and ex vivo [3H]-(+)-PHNO binding sites in rat brain and (2) to compare, using [3H]-(+)-PHNO autoradiography, the ex vivo and in vitro pharmacology of olanzapine, clozapine, risperidone, and haloperidol. Using the D3-selective drug SB277011, we found that ex vivo and in vitro [3H]-(+)-PHNO binding in STR is exclusively due to D2, whereas that in cerebellar lobes 9 and 10 is exclusively due to D3. Surprisingly, the D3 contribution to [3H]-(+)-PHNO binding in the islands of Calleja, ventral pallidum, substantia nigra, and nucleus accumbens was greater ex vivo than in vitro. Ex vivo, systemically administered olanzapine, risperidone, and haloperidol, at doses occupying ~80% D2, did not occupy D3 receptors. Clozapine, which also occupied ~80% of D2 receptors ex vivo, occupied a smaller percentage of D3 receptors than predicted by its in vitro pharmacology. Across brain regions, ex vivo occupancy by antipsychotics was inversely related to the D3 contribution to [3H]-(+)-PHNO binding. In contrast, in vitro occupancy was similar across brain regions, independent of the regional D3 contribution. These data indicate that at clinically relevant doses, olanzapine, clozapine, risperidone, and haloperidol are D2-selective ex vivo. This unforeseen finding suggests that their clinical effects cannot be attributed to D3 receptor blockade.
The Antipsychotics Olanzapine, Risperidone, Clozapine, and Haloperidol Are D2-Selective Ex Vivo but Not In Vitro
пятница, 11 июня 2010 г.
четверг, 10 июня 2010 г.
Мы прервём изложение истории болезни на этом месте и подведём краткие итоги. Нет сомнения, что наш пациент страдает параноидной шизофренией с вербальными псевдогаллюцинациями, вычурными сенестопатиями, при которой навязчивые расстройства, имевшие место в дебюте заболевания, постепенно перерастали в начальные проявления синдрома Кандинского-Клерамбо. Что же такое есть утверждение пациента о наличии у него шизофрении, при том, что она действительно имеет место быть? Да, может показаться при поверхностном взгляде, что мы имеем дело с неординарной личностью, склонной к рефлексии, с грамотным начитанным молодым человеком, блестяще распознавшим диагностировавшим у себя самого психическое заболевание. Был грех, так мы и думали при первом знакомстве с Иваном. Проведя «работу над ошибками», мы осознали, что это далеко не так. Конечно же, дело не в том, верно или нет больной диагностирует у себя ту или иную форму заболевания. Вся динамика состояния, смена одних психопатологических феноменов другими, личностная оценка своего состояния и суждения о прогнозе своего заболевания, постоянные обвинения врачей и медицины в терапевтической несостоятельности и упорное доказывание наличия у себя того, что давно и всем очевидно (психического заболевания – шизофрении), свидетельствует вовсе не о критике, а о формировании ипохондрического бреда, при котором на фоне многолетнего развития многочисленных расстройств восприятия, появления отдельных симптомов синдрома Кандинского-Клерамбо возникает бредовая интерпретация своего состояния, оформленная в психиатрический диагноз, полностью совпадающий с реальностью.
Что такое «расстройство критики»?
Рабочая память
Нарушения памяти традиционно рассматривались практикующими психиатрами как один из важных диагностических признаков, позволяющих дифференцировать «органическое» и «неорганическое». Ни в DSM-IV ни в сегодняшней ее версии DSM-IV-TR (2000), ни в ICD-10 мы не найдем никаких упоминаний о нарушениях памяти как характерном расстройстве при шизофрении. В ICD-10 можно найти лишь замечание о том, что с течением времени при шизофрении может отмечаться определенное когнитивное снижение,[1] а также, что новейшие исследования мозга с помощью СT, PET, MRI показали, что «мы более не знаем что называть «органическим»,[2] и что создатели ICD-10 одно время рассматривали термин «когнитивный» в качестве возможной замены термина «органический», но так и не решились на такую замену.[3]
Может быть, клиницисты и исследователи имеют в виду разные вещи, когда говорят о памяти? Похоже, что так. Авторы отмечают, что предполагаемые нарушения памяти при шизофрении включают в себя, к примеру, дефицит рабочей памяти (working memory), при этом они дают следующее определение working memory: "Working memory is the process of actively holding information in consciousness and manipulating it in service of guiding behavior" (Рабочая память – процесс активного удержания информации в сознании и манипуляции ею для обеспечения управления поведением). Для клинициста такое определение, в особенности вторая его половина, - скорее, определение мышления, чем памяти, и если заранее не определиться в терминах, можно долго и бесплодно дискутировать o том, являются ли, например, галлюцинации нарушением восприятия или мышления, или свидетельствуют ли конфабуляции о нарушениях памяти или мышления, и возможно ли вообще искусственное разделение целостной психики на отельные элементы и т.д.
Критерии расстройства личности, издержки деинституционализации, накопдение лиц с психическими расстройствами в пенитенциарных учреждениях, недобровольная госпитализация
Гиперседация и помрачение сознания после инъекции пролонгированной формы оланзапина
Based on approximately 45,000 olanzapine LAI injections given to 2054 patients in clinical trials through 14 October 2008, post-injection delirium/sedation syndrome occurred in approximately 0.07% of injections or 1.4% of patients (30 cases in 29 patients). Symptomatology was consistent with olanzapine overdose (e.g., sedation, confusion, slurred speech, altered gait, or unconsciousness). However, no clinically significant decreases in vital signs were observed. Symptom onset ranged from immediate to 3 to 5 hours post injection, with a median onset time of 25 minutes post injection. All patients recovered within 1.5 to 72 hours, and the majority continued to receive further olanzapine LAI injections following the event. No clear risk factors were identified.
This exposure is most likely the result of unintended partial intravascular injection or blood vessel injury during the injection (occurring even with proper injection technique) with subsequent seepage of the medication into the vasculature, which would produce higher than intended olanzapine concentrations and symptoms consistent with PDSS.
Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, I: analysis of cases.
Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, II: investigations of mechanism
понедельник, 7 июня 2010 г.
Депрессия и шоколад
study showed that 40 grams of dark chocolate per day reduces the urinary excretion of the stress hormone cortisol and it almost normalizes the stress related differences in energy metabolism and gut microbial activities between participants with low and high anxiety traits. Already after one week metabolic changes were evident in the metabolic profiles of participants compared to the baseline analyses. This became more significant after two weeks of dark chocolate at 40 grams per day. The metabolic changes in both endogenous and gut microbial metabolism were evident.
The carbohydrates in chocolate increase neurotransmitters, such as: serotonin, dopamine and phenylethylamine, which alleviate depression, and give general feelings of well-being. Cocoa also contains M A O inhibitors (monoamine oxidase inhibitors) which help prolong the benefits of neurotransmitters. Cocoa also raises endorphins, which increase pleasure and lessen pain.
The vitamins found in cocoa are:
- Vitamins B1,2,3,5, and 11 which, in conjunction with other vitamins, help release energy from food, and aid the formation of the body's defenses.
- Vitamin D which helps the uptake of calcium and phosphorus, good for teeth and bones.
- Vitamin E has antioxidant properties, helps build muscle, and promotes the production of red blood cells, and protects cell walls.
Most of the studies concluded that the chocolate’s advantages on mood are thought to be short-lived, a momentary band-aid to a bigger difficulty. While chocolate may lift up your mood at first, it rapidly wears off. So, be careful to increase your dose to get the better anti-depressant effect. Otherwise, the positive influence of this “happy food” can be converted for the significant harm to your health.
Chocolate against Stress and Depression
пятница, 4 июня 2010 г.
Новое в механизме действия лития
Though it has been prescribed for over 50 years to treat bipolar disorder, there are still many questions regarding exactly how lithium works. However, in a study appearing in this month's Journal of Lipid Research, researchers have provided solid evidence that lithium reduces brain inflammation by adjusting the metabolism of the health-protective omega-3-fatty acid called DHA.
Uncovering Lithium's Mode of Action
четверг, 3 июня 2010 г.
Рабочие дозы антипсихотиков
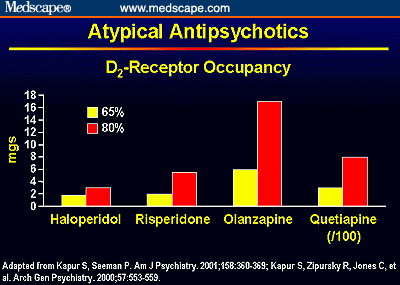
This bar chart illustrates the dose of antipsychotic medications needed to occupy D2-dopamine receptors at 65% (at which point the antipsychotic effect begins) and 80% (at which point EPS begins). The wider dosing margin between these occupancy levels with newer antipsychotics explains the lower EPS liability of these drugs. The quetiapine dose is divided by 100 to fit on the chart; note that it is unknown if quetiapine occupies 80% of D2-dopamine receptors at any dose, hence the low EPS liability of this compound.
Long-term Tolerability of Second-Generation Antipsychotics in Bipolar Disorder
Потенциирование, добавление второго антипсихотика или повышение доз атипичных антипсихотиков
The atypical antipsychotics risperidone, olanzapine, quetiapine, ziprasidone,and aripiprazole have become first-line treatment for schizophrenia because they reduce the positive symptoms of psychosis but do not have a high incidence of extrapyramidal symptoms. However, these agents, like other antipsychotics, may take as long as 16 or more weeks to produce a response, and even with prolonged treatment are unlikely to evoke responses greater than 50% improvement in symptoms. This has led to the experimental use of high atypical antipsychotic doses, antipsychotic polypharmacy, and augmentation with other psychotropic drugs, all of which occur commonly in clinical practice. This article reviews the current evidence for these increasingly common means of treating
schizophrenia and psychosis, with particular emphasis on polypharmacy and augmentation. To date, there are only two controlled studies of antipsychotic polypharmacy involving an atypical antipsychotic; the rest of the data are uncontrolled trials and case reports that describe a mixture of positive and negative findings. One multicenter, double-blind trial shows a faster onset of action when divalproex is added to risperidone or olanzapine than with antipsychotic monotherapy. A small double-blind study demonstrates efficacy when lamotrigine is added to clozapine. The rest of the data on augmentation with anticonvulsants are uncontrolled, and most report adverse effects. With the exception of divalproex, there are currently no compelling data to justify the use of antipsychotic polypharmacy or augmentation. Existing evidence suggests that the best treatments for schizophrenia and psychosis may be long-term trials of a sequence of atypical antipsychotic monotherapies at therapeutic doses.
Increasing the dose in patients with partial responses or breakthrough symptoms is faster and easier than switching to another agent, and it is possible that it would result in improved efficacy. There are also some patients who may be
rapid metabolizers, and thus require higher doses than the average patient. However, although some patients may benefit from higher doses, this method of treatment can
increase the risk of side effects, especially motor side effects. Positron emission tomography (PET) data demonstrate that dopamine 2 (D2) receptor occupancy of 70% is necessary for therapeutic benefits, while occupancy greater than 80% is associated with extrapyramidal symptoms [34]. Doses at the upper end of the recommended range for the first-line antipsychotics may already result in 80% occupancy of D2
receptors in the nigrostriatal pathway [35], so that doses above those ranges are more likely to induce EPS. In particular, the risk of EPS with risperidone is dose-
dependent and may even increase above 4 mg/day
In summary, there is currently no compelling evidence to support long-term antipsychotic polypharmacy. There are few theoretical benefits and many theoretical detriments. Although individual patients may respond to antipsychotic
polypharmacy without side effects, adequate trials have not yet determined the costs versus the benefits of this option.
The evidence for augmentation of atypical antipsychotics varies depending on the particular agent. Controlled studies with conventional antipsychotics suggest that augmentation with benzodiazepines is most likely useful as an acute treatment for patients with agitation and hostility, but controlled studies do not exist for atypical antipsychotics. As mentioned earlier, a multicenter double-blind study demonstrates the safety and efficacy of only one augmenting agent in schizophrenia, namely divalproex [96]. Controlled studies combining divalproex and an atypical antipsychotic also show additive benefits in bipolar disorder, strengthening the appeal of this particular augmenting strategy [167-168]. Unfortunately, there are no controlled data for augmentation of atypical antipsychotics with other anticonvulsants even though this is a frequent and expensive practice, especially with gabapentin. Thus, the evidence currently suggests that divalproex is perhaps the best evidence-based augmentation option when multiple monotherapies fail
A Critical Review of Atypical Antipsychotic Utilization: Comparing
Monotherapy with Polypharmacy and Augmentation
Винпоцетин при деменции
All identified studies were performed before the 1990s and used various terms and criteria for cognitive decline and dementia. The three studies included in the review involved a total of 583 people with dementia treated with vinpocetine or placebo. The reports of these studies did not make possible any differentiation of effects for degenerative or vascular dementia. The results show benefit associated with treatment with vinpocetine 30mg/day and 60 mg/day compared with placebo, but the number of patients treated for 6 months or more was small. Only one study extended treatment to one year. Adverse effects were inconsistently reported and without regard for relationship to dose. The available data do not demonstrate many problems of adverse effects but intention-to-treat data were not available for any of the trials. REVIEWER'S CONCLUSIONS: Although the basic science is interesting, the evidence for beneficial effect of vinpocetine on patients with dementia is inconclusive and does not support clinical use. The drug seems to have few adverse effects at the doses used in the studies. Large studies evaluating the use of vinpocetine for people suffering from well defined types of cognitive impairment are needed to explore possible efficacy of this treatment.
Vinpocetine for cognitive impairment and dementia.
Сосудистая деменция: фармакотерапия
Vascular dementia is a common condition for which there are no effective approved pharmacological treatments available. Absence of effective treatments creates a difficult situation for those suffering from the disease, their caregivers, and healthcare providers. This review will address our current understanding of the mechanisms of nerve cell damage due to ischemia and summarize available clinical trial data on several commonly used compounds including memantine, donepezil, galantamine, rivastigmine, nimodipine, hydergine, nicergoline, CDP-choline, folic acid, as well as such nonpharmacological approaches as validation therapy.
From the studies reviewed here, one may draw several conclusions. First, there are relatively few studies on vascular dementia treatment and no compound has been approved by any regulatory body for treatment of vascular dementia. Second, it appears that there are several compounds with different mechanisms of action that show mild efficacy in improving cognition and even ADLs in patients with vascular dementia. Third, there is one compound (memantine) that has been suggested to act within the confines of the current excitotoxic cell death model, although direct evidence confirming this hypothesis is still lacking. Overall, one could easily conclude that a number of different mechanisms may be at play in ethiopathogenesis of vascular dementia. Vascular conditions aside, nerve cell resistance to injury and our efforts to manipulate it still remains a conundrum, which will require new technologies to solve.
Vascular dementia: Pharmacological treatment approaches and perspectives
среда, 2 июня 2010 г.
Поддерживающие лечение рисперидоном
Objective: Prevention of relapse is the crucial task in the maintenance treatment of schizophrenia. The investigators in this study sought to determine the duration of maintenance treatment needed with the initial therapeutic dose, in contrast to a reduced dose.
Method: In a multicenter open-label, randomized, controlled study, patients with schizophrenia who were clinically stabilized following an acute episode were randomly assigned to a no-dose-reduction group (initial optimal therapeutic dose continued throughout the study), a 4-week group (initial optimal therapeutic dose continued for 4 weeks, followed by a 50% dose reduction that was maintained until the end of the study), or a 26-week group (initial optimal therapeutic dose continued for 26 weeks, followed by a 50% dose reduction until the end of the study). All patients continued until the last recruited patient completed the 1-year follow-up.
Results: Of the 404 patients who met the entry criteria and were randomly assigned, 374 completed the study. The estimated mean time from entry to relapse was 571 days in the 4-week group, 615 days in the 26-week group, and 683 days in the no-dose-reduction group, with estimated relapse rates of 30.5%, 19.5%, and 9.4%, respectively. Patients in the no-dose-reduction group experienced greater reduction in the severity of psychotic symptoms.
Conclusions: Patients who continued to receive the full risperidone dose used for their acute episode had fewer relapses than those who had dose reductions after 4 weeks or 26 weeks during the maintenance period. There was negligible difference in side effects among the three groups.
Risperidone Maintenance Treatment in Schizophrenia: A Randomized, Controlled Trial
Сертралин+налтрексон
More depressed alcohol-dependent patients receiving the sertraline plus naltrexone combination achieved abstinence from alcohol, had delayed relapse to heavy drinking, reported fewer serious adverse events, and tended to not be depressed by the end of treatment.
A Double-Blind, Placebo-Controlled Trial Combining Sertraline and Naltrexone for Treating Co-Occurring Depression and Alcohol Dependence
вторник, 1 июня 2010 г.
Сравнительная эффективность типичных и атипичных антипсихотиков в терапии первого психотического эпизода
Background
There is an ongoing debate about the use of atypical antipsychotics as a first-line treatment for first-episode psychosis.
Aims
To examine the evidence base for this recommendation.
Method
Meta-analyses of randomised controlled trials in the early phase of psychosis, looking at long-term discontinuation rates, short-term symptom changes, weight gain and extrapyramidal side-effects. Trials were identified using a combination of electronic (Cochrane Central, EMBASE, MEDLINE and PsycINFO) and manual searches.
Results
Fifteen randomised controlled trials with a total of 2522 participants were included. No significant differences between atypical and typical drugs were found for discontinuation rates (odds ratio (OR) = 0.7, 95% CI 0.4 to 1.2) or effect on symptoms (standardised mean difference (SMD) = –0.1, 95% CI –0.2 to 0.02). Participants on atypical antipsychotics gained 2.1 kg (95% CI 0.1 to 4.1) more weight than those on typicals, whereas those on typicals experienced more extrapyramidal side-effects (SMD = –0.4, 95% CI –0.5 to –0.2).
Conclusions
There was no evidence for differences in efficacy between atypical and typical antipsychotics, but there was a clear difference in the side-effect profile.
Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis
There is an ongoing debate about the use of atypical antipsychotics as a first-line treatment for first-episode psychosis.
Aims
To examine the evidence base for this recommendation.
Method
Meta-analyses of randomised controlled trials in the early phase of psychosis, looking at long-term discontinuation rates, short-term symptom changes, weight gain and extrapyramidal side-effects. Trials were identified using a combination of electronic (Cochrane Central, EMBASE, MEDLINE and PsycINFO) and manual searches.
Results
Fifteen randomised controlled trials with a total of 2522 participants were included. No significant differences between atypical and typical drugs were found for discontinuation rates (odds ratio (OR) = 0.7, 95% CI 0.4 to 1.2) or effect on symptoms (standardised mean difference (SMD) = –0.1, 95% CI –0.2 to 0.02). Participants on atypical antipsychotics gained 2.1 kg (95% CI 0.1 to 4.1) more weight than those on typicals, whereas those on typicals experienced more extrapyramidal side-effects (SMD = –0.4, 95% CI –0.5 to –0.2).
Conclusions
There was no evidence for differences in efficacy between atypical and typical antipsychotics, but there was a clear difference in the side-effect profile.
Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis
Подписаться на:
Сообщения (Atom)