OBJECTIVE: Despite the clinical observation that antipsychotics can produce negative symptoms, no previous controlled study, to our knowledge, has evaluated this action in healthy subjects. The present study assessed observer-rated and self-rated negative symptoms produced by conventional and second-generation antipsychotics in healthy volunteers. METHOD: The authors used a double-blind, placebo-controlled trial of single doses of haloperidol (5 mg) and risperidone (2.5 mg) in normal subjects. Thirty-two subjects were administered haloperidol, risperidone, and placebo in a random order. Motor variables and observer-rated negative symptoms were assessed after 3–4 hours and subjective negative symptoms and drowsiness after 24 hours. RESULTS: Neither of the active drugs caused significant motor extrapyramidal symptoms after administration. Haloperidol caused significantly more negative signs and symptoms than placebo on the Scale for the Assessment of Negative Symptoms (SANS) and two self-rated negative symptom scales: the Subjective Deficit Syndrome Scale total score and an analog scale that evaluates subjective negative symptoms. Risperidone caused significantly more negative signs and symptoms than placebo on the Brief Psychiatric Rating Scale (BPRS), the SANS, the Subjective Deficit Syndrome Scale total score, and the analog scale for subjective negative symptoms. After control for drowsiness, risperidone but not haloperidol produced more negative symptoms than placebo on the BPRS and the SANS. Significance was lost for the subjective negative symptoms with both drugs. CONCLUSIONS: Single doses of both haloperidol and risperidone produce negative symptoms in normal individuals. Drowsiness may be an important confounding factor in the assessment of negative symptoms in antipsychotic trials.
Показаны сообщения с ярлыком седация. Показать все сообщения
Показаны сообщения с ярлыком седация. Показать все сообщения
четверг, 26 января 2012 г.
Исследование вторичной негативной симптоматики при приёме антипсихотиков
четверг, 10 июня 2010 г.
Гиперседация и помрачение сознания после инъекции пролонгированной формы оланзапина
Based on approximately 45,000 olanzapine LAI injections given to 2054 patients in clinical trials through 14 October 2008, post-injection delirium/sedation syndrome occurred in approximately 0.07% of injections or 1.4% of patients (30 cases in 29 patients). Symptomatology was consistent with olanzapine overdose (e.g., sedation, confusion, slurred speech, altered gait, or unconsciousness). However, no clinically significant decreases in vital signs were observed. Symptom onset ranged from immediate to 3 to 5 hours post injection, with a median onset time of 25 minutes post injection. All patients recovered within 1.5 to 72 hours, and the majority continued to receive further olanzapine LAI injections following the event. No clear risk factors were identified.
This exposure is most likely the result of unintended partial intravascular injection or blood vessel injury during the injection (occurring even with proper injection technique) with subsequent seepage of the medication into the vasculature, which would produce higher than intended olanzapine concentrations and symptoms consistent with PDSS.
Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, I: analysis of cases.
Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, II: investigations of mechanism
четверг, 20 мая 2010 г.
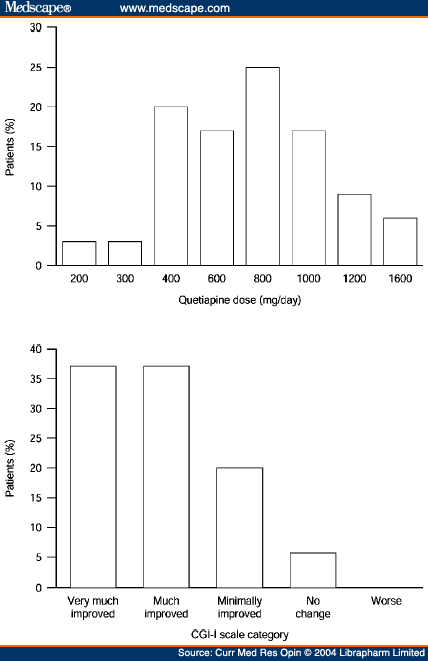
Кветиапин: эффективность и переносимость доз
"Quetiapine has a wide clinical dose range that extends up to 750mg/day (up to 800 mg/day in the USA and Canada).[21,22] Although administration is usually twice-daily,[23] a pilot study in patients with schizophrenia or schizoaffective disorder found similar levels of clinical efficacy in patients administered quetiapine on a once-or twice-daily basis.[24] Moreover, our clinical experience has suggested that a more rapid initiation schedule than that described in current prescribing information (300 mg/day by Day 4; 300-400 mg/day by Day 4 in the USA and Canada),[21] with a higher target dose, may be more appropriate in hospitalised adult and adolescent patients with an acute exacerbation of schizophrenia in order to achieve rapid control of symptoms. Some evidence supporting this assertion can be found in a poster that described a randomised, multicentre, double-blind, pilot study of hospitalised patients with acute schizophrenia who had their doses of quetiapine (twice-daily oral administration) titrated to 400 mg by Days 2, 3 or 5, without any increased risk of adverse events compared with classic initiation.[25] The overall frequency of adverse events was similar among the three initiation groups and somnolence was experienced by fewer than 15% of patients in each initiation group; indeed, the lowest incidence of somnolence (8%) was observed in the group of patients using the fastest initiation scheme (400 mg by Day 2). Throughout the study, patient vital signs and laboratory values were similar among the three initiation groups. The somnolence observed in these quetiapine-treated patients is likely to be a result of histamine H1 antagonism to which tolerance usually develops over time. Experience has shown that somnolence with quetiapine is not dose-related, most cases are mild and transient (usually dissipating after approximately 2 weeks), and that the incidence of somnolence does not increase over time. These data support our clinical experience of initiating quetiapine at 200mg/day in the inpatient setting and increasing the dose in increments of 200 mg/day."
Dosing of quetiapine and Clinical Global Improvement scores in a small study of patients with schizophrenia, schizoaffective disorder or other psychotic disorders.
Managing Acute Exacerbations of Schizophrenia: Focus on: Influence of Tolerability on Treatment Selection
Dosing of quetiapine and Clinical Global Improvement scores in a small study of patients with schizophrenia, schizoaffective disorder or other psychotic disorders.
Managing Acute Exacerbations of Schizophrenia: Focus on: Influence of Tolerability on Treatment Selection
Подписаться на:
Сообщения (Atom)