APs suppress induction of pro-inflammatory cytokines. It is well established that psychotic episodes of schizophrenia are associated with neuroinflammation and elevations of cytokines such as interleukin 1 (IL-1), IL-6, tumor necrosis factor (TNF-α), and interferon gamma (IFN-γ). These inflammatory biomarkers are released by microglia, which are rapidly activated by psychosis and mediate brain tissue damage during psychosis. APs’ rapid inhibitory action on pro-inflammatory cytokines obviously is neuroprotective.Beyond dopamine: The ‘other’ effects of antipsychotics
APs suppress immune-inflammatory pathways. This occurs with atypical agents but not haloperidol and results in decreased IL-1β and IL-6 and transforming growth factor-β.
APs significantly decrease levels of neurotoxic tryptophan catabolites (TRYCATS) such as 3-OHK and QUIN, which mediate the immune-inflammatory effects on neuronal activity. APs also increase levels of neuroprotective TRYCATS such as kynurenic acid.
APs activate cholesterol-transport proteins such as apolipoprotein E (APOE).6 This implies that APs may improve low levels of APOE observed during psychosis and decrease myelination abnormalities and mitigate impairment of synaptic plasticity.
APs increase neurotrophic growth factors that plummet during psychosis, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor. This beneficial effect is seen with SGAs but not first-generation APs (FGAs) and is attributed to strong serotonin 5HT-2A receptor antagonism by SGAs.
SGAs but not FGAs significantly increase the number of newly divided cells in the subventricular zone by 200% to 300%. This enhancement of neurogenesis and increased production of progenitor cells that differentiate into neurons and glia may help regenerate brain tissue lost during psychotic episodes.
Various SGAs have neuroprotective effects:
Clozapine has neuroprotective effects against liposaccharide-induced neurodegeneration and reduces microglial activation by limiting production of reactive oxygen species (free radicals).
Aripiprazole inhibits glutamate-induced neurotoxicity and, in contrast to haloperidol, increases BDNF, glycogen synthase kinase (GSK)-β, and the anti-apoptotic protein Bcl-2.
Olanzapine increases BDNF, GSK-3β, and β-catenin, increases mitosis in neuronal cell culture, and protects against neuronal death in cell cultures that lack nutrients (which fluphenazine or risperidone do not).
Paliperidone demonstrates a higher antioxidant effect than any other SGA and clearly is better than haloperidol, olanzapine, or risperidone in preventing neuronal death when exposed to hydrogen peroxide.
Quetiapine, ziprasidone, and lurasidone have inhibitory effects on nitric oxide release. Quetiapine, but not ziprasidone, inhibits TNF-α.
Ziprasidone inhibits apoptosis and microglial activation and synthesis of nitric oxide and other free radicals.
Lurasidone increases BDNF expression in the prefrontal cortex of rodents.
Показаны сообщения с ярлыком кветиапин. Показать все сообщения
Показаны сообщения с ярлыком кветиапин. Показать все сообщения
четверг, 6 июня 2013 г.
Не связанные с дофамином механизмы действия антипсихотиков
вторник, 2 октября 2012 г.
Целесообразность применения монотерапии кветиапином для лечения депрессии
BackgroundSchizophrenia and bipolar depression trials suggest that quetiapine may have an antidepressant effect.ObjectivesThis meta-analysis aimed to determine the efficacy, acceptability and tolerability of quetiapine treatment for major depressive disorder (MDD). Only the randomized controlled trials (RCTs) comparison between quetiapine and placebo were included. The authors searched such clinical trials carried out between 1991 and February 2012.Data sourcesMEDLINE, EMBASE, CINHL, PsycINFO and Cochrane Controlled Trials Register were searched in February 2012. Study populations comprised adults with MDD or major depression.Study eligible criteria, participants and interventionsEligible studies were randomized, placebo-controlled trials of quetiapine monotherapy carried out in adults with MDD and presenting endpoint outcomes relevant to: i) depression severity, ii) response rate, iii) overall discontinuation rate, or iv) discontinuation rate due to adverse events. No language restriction was applied.Study appraisal and synthesis methodsAll abstracts identified by the electronic searches were examined. The full reports of relevant studies were assessed, and the data of interest were extracted. Based on the Cochrane methods of bias assessment, risks of bias were determined. The studies with two risks or less were included. The efficacy outcomes were the mean change scores of depression rating scales, the overall response rate, and the overall remission rates. The overall discontinuation rate was considered as a measure of acceptability. The discontinuation rate due to adverse events was a measure of tolerability. Relative risks (RRs) and weighted mean differences (WMDs) with 95% confidence intervals (CIs) were computed by using a random effect model.ResultsA total of 1497 participants in three RCTs were included. All trials examined the quetiapine extended-release (XR). The pooled mean change scores of the Montgomery-Asberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating Scale (HAM-D) of the quetiapine-treated group were higher than those of the placebo-treated group with the WMDs (95%CI) of -3.37 (-3.95, -2.79) and -2.46 (-3.47, -1.45), respectively. All studies defined the response and remission as >= 50% reduction of the MADRS total score and the MADRS total score of <=8 at endpoint, respectively. The overall response and remission rates were significantly greater in the quetiapine-treated group with RRs (95%CIs) of 1.44 (1.26, 1.64) and 1.37 (1.12, 1.68), respectively. The pooled discontinuation rate was not significantly different between groups with an RR (95%CI) of 1.16 (0.97, 1.39). The pooled discontinuation rate due to adverse event was greater in the quetiapine group with an RR (95%CI) of 2.90 (1.87, 4.48). With respect to sleep time, the pooled mean change Pittsburgh Sleep Quality Index (PSQI) scores of the quetiapine-treated group was also significantly higher than that of the placebo-treated group [WMD (95%CI) of -1.21 (-1.81, -0.61)].LimitationsVariety of quetiapine XR doses and the small number of RCTs were key limitations of this meta-analysis.ConclusionsBased on the limited evidence obtained from three RCTs, quetiapine XR is effective for adult patients with MDD. The high dropout rate due to adverse events suggests that some MDD patients may not be able to tolerate quetiapine XR. Due to the balance of its efficacy benefit and risk of side effects, as the overall discontinuation rate shown, the acceptability of this agent is not more than placebo. These results should be viewed as the very preliminary one. Further studies in this area are warranted.
понедельник, 1 октября 2012 г.
Отсутствие долгосрочной противотревожной эффективности потенцирования кветиапином в предварительном исследовании
BackgroundComorbid anxiety symptoms,in patients with a primary anxiety disorder or a mood disorder, leads to poor patient outcomes and burdens the healthcare system. This pilot study evaluated the feasibility of extended-release quetiapine fumarate (quetiapine XR) for the treatment of patients with either a primary anxiety disorder or a mood disorder with comorbid anxiety symptoms compared to a placebo, as an adjunct to antidepressant therapy.MethodsThirty-nine patients with a diagnosis of a primary anxiety disorder or a mood disorder with comorbid anxiety symptoms were enrolled in this study. Patients with a stable dose of antidepressant therapy were randomized according to a 2:1 probability of receiving either quetiapine XR or a placebo adjunctive treatment for 8 weeks. The efficacy was assessed by the Hamilton Anxiety Rating Scale (HAM-A) and the Clinical Global Impression of severity (CGI-S) score at baseline, week 1, 4, and 8.ResultsA total of 35 patients were included in this intention-to treat (ITT) population for the efficacy analysis (quetiapine XR: 22 patients; placebo: 13 patients). At week 4, statistically significant differences were observed on both the HAM-A score (p = 0.003) and the CGI-S score (p = 0.025), favouring the quetiapine XR (-13.00 +/- 4.14) compared to placebo (-6.63 +/- 5.42). However, no statistically significant difference was observed between the two groups with regard to changes from the baseline to week 8 on the HAM-A score (p = 0.332) or the CGI-S score (p = 0.833).ConclusionsAugmentation of antidepressant treatment with quetiapine XR did not result in clinical improvement according to the outcome measure of anxiety using the HAM-A and CGI-S scores at week 8, among the patients with either a primary anxiety disorder or a mood disorder with comorbid anxiety symptoms. However, treatment with quetiapine XR as an adjunct to antidepressant therapy appeared to provide a short-term benefit at 4 weeks. Further study is needed with a larger sample size, randomized controlled design and control of the dosage prescribed.
Quetiapine fumarate augmentation for patients with a primary anxiety disorder or a mood disorder: a pilot study
суббота, 2 июня 2012 г.
Арипиразол, кветиапин и оланзапин в терапии биполярной депрессии
Seven published papers were identified on the use of aripiprazole, olanzapine and quetiapine. Internal validity of the trials was fairly good, external validity only moderate. Different outcome measures of efficacy and safety were assessed. When the individual trials were looked at, quetiapine and to a lesser extent olanzapine demonstrated significant improvement in MADRS (Montgomery–Åsberg Depression Rating Scale) total scores. This was not demonstrated for aripiprazole. Efficacy was hampered by adverse events, such as weight gain, akathisia and somnolence/sedation. Both clinical heterogeneity of the included trials and statistical heterogeneity of the meta-analytic data were considerable. The number of quetiapine trials was disproportionate to the number of trials of aripiprazole and olanzapine. Further research is needed to assess differential efficacy of the different SGAs and their use in clinical practice.
Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis
четверг, 1 сентября 2011 г.
Сравнение антидепрессивной эффективности антипсихотиков у психотических больных
There was no substantial difference in anti-depressive effectiveness among olanzapine, quetiapine, risperidone or ziprasidone in this clinically relevant sample of patients acutely admitted to hospital for symptoms of psychosis. Based on our findings we can make no recommendations concerning choice of any particular SGA for targeting symptoms of depression in a patient acutely admitted with psychosis.Anti-depressive effectiveness of olanzapine, quetiapine, risperidone and ziprasidone: a pragmatic, randomized trial.
вторник, 26 апреля 2011 г.
Метаболические расстройства коморбидные с БАР

Chronic stress, which patients experience during both the manic and the depressive phases of bipolar disorder, is associated with increased cortisol levels, lack of cortisol suppression, and changes in hypothalamic-pituitary-adrenal axis responses. This metabolic dysregulation may increase insulin resistance and can lead to hyperglycemia, increased oxidative stress, metabolic syndrome, and atherosclerosis. In addition, patients with bipolar illness have increased activity of the sympathetic nervous system, which may also lead to insulin resistance, metabolic syndrome, and increased risk of sudden cardiac death.2
Depressive syndromes may be neurotoxic. Abnormalities in cellular plasticity, cellular resilience, and intracellular signaling, as well as alterations in the size, shape, and density of neurons and glia, have been found. Studies employing neuroimaging and neuropsychological tests have demonstrated abnormalities in brain morphology and function in patient populations with depressive syndromes and in those with diabetes. Common physiologic mechanisms have been implicated, including insulin-glucose homeostasis, immuno-inflammatory processes, and oxidative stress mechanisms.
Metabolic Comorbidities in Patients With Bipolar Disorder
суббота, 5 марта 2011 г.
четверг, 17 февраля 2011 г.
Риск обострения при отмене препаратов через год после первого эпизода и при противорецедивной терапии кветиапином
Participants 178 patients with first episode psychosis who had received at least one year of antipsychotic drug treatment between September 2003 and July 2006 and had no positive symptoms of psychosis.
Interventions Patients received either maintenance treatment with quetiapine (400 mg/day) or placebo and were followed up for the next 12 months or until a relapse occurred.
Main outcome measure Relapse assessed monthly and defined as re-emergence of psychotic symptoms (delusions, conceptual disorganisation, hallucinations, suspiciousness, and unusual thought content) according to predefined thresholds.
Results 178 patients were randomised (89 to quetiapine and 89 to placebo). The Kaplan-Meier estimate of the risk of relapse at 12 months was 41% (95% confidence interval 29% to 53%) for the quetiapine group and 79% (68% to 90%) for the placebo group (P<0.001). Although quetiapine was generally well tolerated, the rate of discontinuation due to adverse or serious adverse events was greater in the quetiapine group (18%; 16/89) than in the placebo group (8%; 7/89) (relative risk 2.29, 95% confidence interval 0.99 to 5.28; χ2=3.20, df=1; P=0.07).
Conclusion In a group of asymptomatic patients with first episode psychosis and at least one year of previous antipsychotic drug treatment, maintenance treatment with quetiapine compared with placebo resulted in a substantially lower rate of relapse during the following year.
Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial
среда, 9 февраля 2011 г.
Случай ухудшения маниакальной симптоматики при приёме низких доз кветиапина
The mechanism of antidepressant action of quetiapine is unclear. However, it has been suggested that its antidepressant activity is mediated by its metabolite N-Desalkylquetiapine, which leads to norepinephrine reuptake transporter inhibition and partial serotonin 1A agonism.4 A speculation may be that slow clearance of the metabolites as an age effect or genetic trait in this case led to very high levels of N-Desalkylquetiapine potentiating quetiapine's antidepressant effect and leading to worsening of mania. Moreover, a positron emission tomography (PET) study using quetiapine 750 mg or 450 mg/day found that there was no D2 receptor occupancy at the low dose of quetiapine, while 5HT2A receptor occupancy was consistently high.5 Despite the normal head CT scan, brain-aging related neurotransmitter changes and therefore different medication effects could be considered. Calabrese et al.1 reported an incidence of treatment-emergent mania 2.2% with 600 mg/day of quetiapine and 3.9% with 300 mg/day of quetiapine. The absence of dopaminergic receptors blockade and the high affinity for serotonergic receptors at lower doses, may explain quetiapine's antidepressant activity and worsening mania in case of slow titration in manic patients. In this case, geriatrician treatment practice of "start low and go slow" raised questions.
Worsening Mania Associated With Slow Increase of Quetiapine Dose
пятница, 14 января 2011 г.
Эффективность атипичных антипсихотиков при делирии
Haloperidol is the mainstay of delirium treatment.8 Compared with atypical antipsychotics in delirium treatment, haloperidol doses < 3.5 mg/d have not been associated with an increase in extrapyramidal symptoms (EPS).9
Although not devoid of side effects, atypical antipsychotics are an alternative to haloperidol.8,10 This article briefly summarizes the current evidence on the use of atypicals for treating delirium.
Evidence for antipsychotics
Haloperidol has been the antipsychotic of choice for treating delirium symptoms. It is recommended by the Society of Critical Care Medicine7 and is regarded as safe, cost-effective, and efficacious for delirium5 despite a risk of dose-related EPS and potential cardiac conduction alterations.5,14
Risperidone is not indicated for treating delirium but is one of the most extensively studied atypical antipsychotic alternatives to haloperidol. Evidence consisting primarily of case reports has illustrated the potential efficacy of risperidone in treating delirium (Table 2).10,15-19
Clinical Point
In a small double-blind, randomized trial, risperidone was effective but not significantly more so than low-dose haloperidol
In 2004, Parellada et al17 observed significant mean improvements in all measures (Delirium Rating Scale [DRS], Mini-Mental State Exam [MMSE], positive subscale of the Positive and Negative Syndrome Scale [PANSS-P], and Clinical Global Impressions scale [CGI]) in 64 delirium patients treated with risperidone. In a 2004 double-blind trial of 28 delirium patients randomly assigned to risperidone or haloperidol, risperidone was effective but not significantly more efficacious than low-dose haloperidol for acute delirium treatment.18
Advantages of using risperidone include its lack of anticholinergic effects. Potential side effects include dose-related EPS and weight gain, which were observed in patients with schizophrenia and other psychotic disorders and dementia-related behavioral disorders.20,21
Olanzapine. Much like risperidone, olanzapine’s use in delirium is relatively well described in the literature (Table 3).22-24 In a randomized, placebo-controlled study comparing olanzapine with haloperidol, 175 patients were treated for 7 days with olanzapine, haloperidol, or placebo. Olanzapine and haloperidol showed significantly greater DRS score improvement than placebo.24 There was no difference between olanzapine and haloperidol outcomes; however, olanzapine showed significant improvement by days 2 and 3 compared with haloperidol. Haloperidol was associated with a significantly higher rate of dystonia compared with olanzapine.
Olanzapine carries a risk of anticholinergic effects. This can be a drawback, especially in patients such as Ms. B whose delirium has an anticholinergic component. Olanzapine is available in an IM formulation, which can be an advantage when addressing agitation and medical comorbidities of delirium.
Quetiapine. Case reports have suggested quetiapine is effective for delirium (Table 4).10,25-27 In a prospective, open-label trial, Sasaki et al26 treated 12 delirium patients with a single bedtime dose of quetiapine. All patients achieved remission within several days of beginning quetiapine, and the drug was well tolerated with no detected EPS or excessive sedation.
Clinical Point
Quetiapine reduced delirium duration and agitation in a small double-blind randomized trial of adult ICU patients
In 2010 Devlin et al27 reported on the efficacy and safety of quetiapine in a prospective double-blind, placebo-controlled study of 36 adult ICU patients. Compared with those receiving placebo, patients taking quetiapine had a statistically significant shorter time to first resolution of delirium, reduced duration of delirium, and less agitation as measured by the Sedation-Agitation Scale. Mortality, ICU length of stay, and incidence of QTc prolongation did not differ, but patients treated with quetiapine were more likely to be discharged home or to rehabilitation and to have more somnolence. Quetiapine’s side effect profile includes a low occurrence of EPS, sedation, and dose-related anticholinergic effects.25
Ziprasidone. The literature on ziprasidone for delirium so far is limited to a few anecdotal case reports (Table 5).28-31 In 2002, Leso and Schwartz28 successfully used ziprasidone to treat delirium in a patient with human immunodeficiency virus and cryptococcal meningitis. Ziprasidone was chosen for its lack of sedating effects and low EPS risk. The patient experienced significant clearing of his delirium and lowering of his DRS score. Ziprasidone eventually was discontinued because a fluctuating QTc interval associated with comorbid electrolyte imbalances—a potential drawback to ziprasidone.
In the case of Ms. B, ziprasidone appeared to be efficacious; however, improvement in her medical condition, rather than ziprasidone treatment, is the most likely explanation for the resolution of her delirium symptoms.
Aripiprazole. Alao et al30 reported on 2 delirium patients treated with 30 mg and 15 mg aripiprazole; improvement was monitored using the MMSE and DRS (Table 5).28-31 In both cases, confusion, disorientation, and agitation improved within 7 days of treatment. In the first case, the patient’s MMSE score improved from 5 to 28 and his DRS score decreased from 28 to 6. The second patient’s MMSE score improved from 7 to 27 and her DRS score went from 18 to 6.
Straker et al31 reported on 14 delirium patients treated with aripiprazole. Twelve patients had a ≥50% reduction in DRS, Revised-98 scores, and 13 showed improvement on CGI scores. The rate of adverse side effects was low. Three patients had prolonged QTc interval, but no patients developed arrhythmia or discontinued aripiprazole.
Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?
среда, 29 декабря 2010 г.
Антипсихотики второго поколения в терапии депрессии
Abstract
Purpose of review The aim of this systematic review was to examine the efficacy and safety of second-generation antipsychotics (SGAs) in nonpsychotic major depressive disorder (MDD).
Recent findings In MDD, SGA monotherapy or adjunctive therapy to conventional antidepressants showed rapid onset of antidepressant efficacy. Although maintenance data are limited, quetiapine monotherapy, risperidone adjunctive therapy, and amisulpride adjunctive therapy significantly delayed the time to relapse as compared with placebo. In general, extrapyramidal symptoms appeared to be low with SGAs, but a higher incidence of akathisia was observed with aripiprazole. An elevated risk of weight gain was observed with olanzapine–fluoxetine combination, risperidone, aripiprazole, and quetiapine compared with placebo. At present, there are insufficient data to confidently distinguish between different SGAs in the treatment of MDD. A recent meta-analysis found that adjunctive SGAs were significantly more effective than placebo, but differences in efficacy were not identified among the studied agents, nor were outcomes affected by trial duration or the method of establishing treatment resistance.
Summary Both SGA monotherapy and adjunctive therapy showed greater efficacy in the treatment of MDD than placebo, but augmentation is more widely utilized in treatment-resistant depression. Clinicians should routinely monitor for cardiometabolic side-effects and extrapyramidal symptoms during SGA therapy.
Quetiapine
The efficacy and safety of QTP-XR monotherapy in the treatment of MDD were evaluated in two 8-week, placebo-controlled RCTs.[7••,8•] In the first trial, QTP-XR 150 or 300 mg/day and duloxetine 60 mg/day were compared with placebo.[7••] All active treatment arms demonstrated significant improvement in Montgomery–Asberg Depression Rating Scale (MADRS)[27] total scores compared with that of placebo at week 6. Significant improvement in depressive symptoms occurred at the end of week 1 with both QTP-XR 150 mg/day (−8.4, P < 0.01) and 300 mg/day (−8.2, P < 0.01) compared with placebo (−6.0), but not duloxetine 60 mg/day (−6.8, P = 0.30). At study endpoint (week 6), remission rates (MADRS ≤ 8) were significantly higher in the QTP-XR 300 mg/day (32.0%, P < 0.05) and duloxetine 60 mg/day groups (31.9%, P < 0.05) vs. placebo (20.4%), but not for QTP-XR 150 mg/day (26.5%, P = 0.27).
Sulpiride and Amisulpride
Apart from QTP-XR, SLP and ASLP are the only other SGAs that have been studied as monotherapy treatment for MDD in placebo-controlled trials. A moderately sized (n = 88) study found greater reduction in the 21-item Hamilton Depression Rating Scale (HAM-D-21)[28] total score from baseline to endpoint in the SLP group (−10, P = 0.0007) than in placebo (−8). The only RCT of ASLP in the acute treatment of MDD compared a fixed dosage of ASLP 50 mg/day (n = 136) with paroxetine 20 mg/day (n = 136).[11] No statistically significant differences occurred between the two treatments, but a placebo group was not included to establish internal validity.[11] A long-term (6-month), fixed-dosage, placebo-controlled RCT in mild or moderate MDD or dysthymia compared the efficacy and safety of ASLP (50 mg/day) with imipramine (100 mg/day) and placebo.[12] Analysis of the primary outcome showed the mean change in MADRS total scores in both active treatment arms was significantly larger than that of placebo, although remission rates did not reach the level of statistical significance.
Second-generation Antipsychotics in Major Depressive Disorder: Update and Clinical Perspective
понедельник, 22 ноября 2010 г.
Сравнение атипичных антипсихотиков



For example, when switching from a tightly binding anticholinergic or antihistaminergic medication (eg, olanzapine, quetiapine, clozapine) to one with less anticholinergic or antihistaminergic affinity (eg, aripiprazole, risperidone, ziprasidone), often transient rebound anxiety, insomnia, agitation and restlessness can occur. In addition, when switching from a tighter D2 binding agent to a looser-binding agent (eg, from risperidone to clozapine or quetiapine) or, particularly, to a partial dopamine agonist (eg aripiprazole) dopamine rebound symptoms, such as often transient worsening of psychosis, mania or aggression/agitation, can occur. A pharmacokinetic dopamine rebound may also occur when switching from a short half-life antipsychotic to a longer half-life antipsychotic (Table 1).4
The abrupt switch has the greatest potential for rebound and withdrawal phenomena. Even the conventional cross-titration can lead to problems when the pre-switch antipsychotic has a shorter half life and/or blocks more tightly cholinergic, histaminergic or dopaminergic receptors than the post-switch antipsychotic. Rebound phenomena can be minimized by avoiding abrupt or fast switching when the pre- and post-switch receptor affinities and/or half-lives differ considerably. Instead, an overlapping or “plateau” switch should be used. This consists of decreasing the pre-switch antipsychotic slowly (eg, 25–50% every 5 half-lives) and only after the post-switch antipsychotic has reached steady state (ie, ≤5 half lives on target dose). Adding calming medications during the switch period, such as benzodiazepines, antihistamines or sleep aides, can also minimize rebound phenomena.
A number of non-antipsychotic augmentation strategies have also been tested in schizophrenia patients with insufficient response to antipsychotic monotherapy. Of these, lithium,16 carbamazepine,17 and beta blockers18 were not superior to placebo when added to an antipsychotic. Similarly, benzodiazepine19 and valproate augmentation20 also did not show long-term superiority compared to placebo, although both agents might speed up the initial response. Although two large-scale studies showed no superiority of lamotrigine augmentation of antipsychotics compared to placebo,21 a meta-analysis demonstrated significant superiority regarding global ratings of psychopathology, positive and negative symptom change, as well as study-defined response when outcomes of patients were combined in whom lamotrigine was added to clozapine.22 This, however, has not been verified in a prospective study.
ECT augmentation has also been shown to be superior, both for acute efficacy and in maintenance treatment, when added to antipsychotic monotherapy in patients who have failed antipsychotic monotherapy.23
One meta-analysis suggested that augmentation of antipsychotics with antidepressants may be more helpful than placebo for schizophrenia patients with predominantly negative symptoms.24 Larger, validating studies are needed, however, and specific effects on negative symptoms need to be distinguished from proven effects of antidepressants on depressive symptoms in schizophrenia patients.25
Practical Dosing Strategies in the Treatment of Schizophrenia: Part 2 - Switching and Combining Antipsychotics
вторник, 26 октября 2010 г.
Антипсихотики второго поколения в сравнении с перфеназином в терапии депрессивного синдрома у больных шизофренией
Second-generation antipsychotics (SGAs), also known as aytypicals, are not superior to the first-generation antipsychotic perphenazine in treating depression in patients with chronic schizophrenia
Although a significant improvement in depressive symptoms was found in all treatment groups over time, subanalyses found that quetiapine was significantly more effective than risperidone in the patients having a major depressive episode (MDE)
No Evidence to Support Recommendation to Use Atypicals Over First-Generation Antipsychotic
вторник, 19 октября 2010 г.
Normally, after eating, your body uses carbohydrate as the main energy source. After a long time hungry, it switches to fat. Zyprexa made the body use fat all the time
SUMMARY: A class effect, to varying degrees; and eating less may not help.
1. Food intake was the same between controls and Zyprexaers. You get these effects even if you eat the same.
2. This effect is shared by other atypicals, in a predictable fashion:
In the fed state, Zyprexa and Clozaril do a massive conversion to fat utilization, Risperdal a medium, and sulpiride minimal covnersion.
In the fasting state:
Geodon has a lesser effect than Zyprexa, and appears to normalize; Abilify and Haldol seem close to normal.
3. These effects are consistent with Lilly's own studies that the majority of weight gain happens in the first month, and not suddenly after a year of use.
4. There is still a hunger component to weight gain that is separate from the metabolic effect. Some drugs will make you hungry, change your metabolism, or some mixture of the two. Hunger appears to be a H1 mediated process (Seroquel, Zyprexa, Clozaril, Remeron, Paxil>Prozac, etc.)
5. The immediate clinical consequence of this information is probably (paradoxically) to tell the patients to eat less sugar.
Unless you dramatically cut fat out of your diet, the body will still churn through what fat you do eat at the expense of carbohydrate. Better, and easier, to reduce the carb load that lingers in your body (and likely ultimately gets stored.)
Why Zyprexa (And Other Atypical Antipsychotics) Make You Fat
пятница, 15 октября 2010 г.
пролонгированная форма рисперидона vs кветиапин
Chronic management of schizophrenia and schizoaffective disorders is frequently complicated by symptomatic relapse. An open-label, randomized, active-controlled, 2-year trial evaluated 710 patients with schizophrenia or related disorders who were switched from stable treatment with oral risperidone, olanzapine, or conventional neuroleptics to risperidone long-acting injectable (RLAI) or oral quetiapine. Primary effectiveness evaluation was time-to-relapse. Safety evaluations included adverse events (AEs) reported for the duration of the study, Extrapyramidal Symptom Rating Scale (ESRS), clinical laboratory tests, and vital signs. A total of 666 patients (n=329 RLAI, n=337 quetiapine) were evaluable for effectiveness measures. Baseline demographics were similar between treatment groups. Kaplan–Meier estimate of time-to-relapse was significantly longer with RLAI (p < 0.0001). Relapse occurred in 16.5% of patients with RLAI and 31.3% with quetiapine. RLAI and quetiapine were both safe and well tolerated. Weight gain affected 7% of patients with RLAI and 6% with quetiapine, with mean end point increases of 1.25±6.61 and 0±6.55 kg, respectively. There were no significant between-group differences in weight gain. ESRS total scores decreased similarly after randomization to either RLAI or quetiapine. Extrapyramidal AEs occurred in 10% of patients with RLAI and 6% with quetiapine. Treatment-emergent potentially prolactin-related AEs were reported in 15 (5%) patients with RLAI and 5 (2%) patients with quetiapine; hyperprolactinemia was reported in 43 (13.1%) patients with RLAI and 5 (1.5%) patients with quetiapine. Somnolence occurred in 2% of patients with RLAI and 11% with quetiapine. To our knowledge, this is the first report of a randomized clinical trial directly comparing relapse prevention with a second-generation long-acting injectable antipsychotic and oral therapy. Time-to-relapse in stable patients with schizophrenia or schizoaffective disorder was significantly longer in patients randomized to RLAI compared with those randomized to oral quetiapine. Both antipsychotics were generally well tolerated.
Relapse Prevention in Schizophrenia and Schizoaffective Disorder with Risperidone Long-Acting Injectable vs Quetiapine: Results of a Long-Term, Open-Label, Randomized Clinical Trial
пятница, 24 сентября 2010 г.
Подбор доз антипсихотиков второго поколения
Drug-drug interactions or genetic variability may require using doses different from those recommended for atypical antipsychotics. Dosage alterations of olanzapine and clozapine, dependent on cytochrome P450 1A2 (CYP1A2) for clearance, and quetiapine, dependent on cytochrome P450 3A (CYP3A), may be necessary when used with other drugs that inhibit or induce their metabolic enzymes. Smoking cessation can significantly increase clozapine, and perhaps olanzapine, levels. Ziprasidone pharmacokinetic drug-drug interactions are not likely to be important. Genetic variations of cytochrome P450 2D6 (CYP2D6) and drug-drug interactions causing inhibition (CYP2D6 and/or CYP3A) or induction (CYP3A) may be important for risperidone, and perhaps for aripiprazole, dosing. Adding inhibitors may cause side effects more easily in drugs with a narrow therapeutic window, such as clozapine or risperidone, than in those with a wide therapeutic window, such as olanzapine or aripiprazole. Adding inducers may be associated with a gradual development of lost efficacy.
The Dosing of Atypical Antipsychotics
вторник, 21 сентября 2010 г.
Смертность пациентов с деменцией получающих антипсихотические препараты
They found that no antipsychotic was associated with greater long-term mortality, but that haloperidol, olanzapine, and risperidone, but not quetiapine, were associated with a short-term increase in mortality during the first 30 days of prescribing.
Are Commonly Prescribed Antipsychotics Associated With Greater Mortality in Patients With Dementia?
четверг, 3 июня 2010 г.
Рабочие дозы антипсихотиков
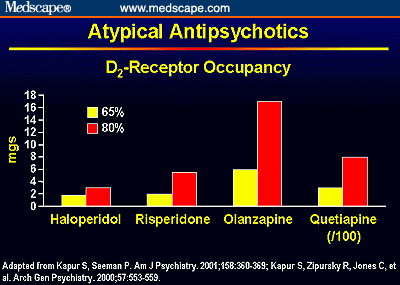
This bar chart illustrates the dose of antipsychotic medications needed to occupy D2-dopamine receptors at 65% (at which point the antipsychotic effect begins) and 80% (at which point EPS begins). The wider dosing margin between these occupancy levels with newer antipsychotics explains the lower EPS liability of these drugs. The quetiapine dose is divided by 100 to fit on the chart; note that it is unknown if quetiapine occupies 80% of D2-dopamine receptors at any dose, hence the low EPS liability of this compound.
Long-term Tolerability of Second-Generation Antipsychotics in Bipolar Disorder
четверг, 20 мая 2010 г.
Кветиапин: эффективность и переносимость доз
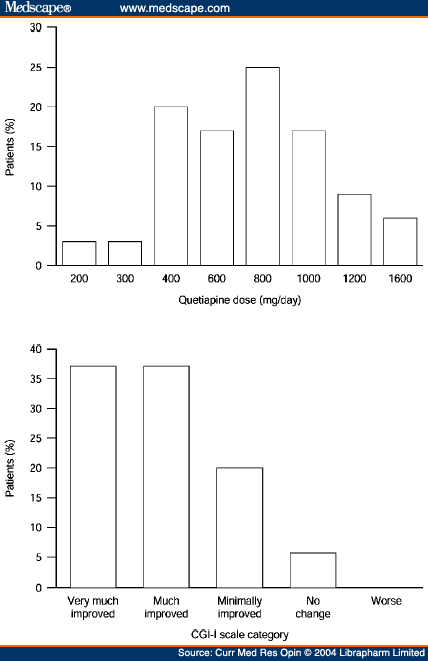
"Quetiapine has a wide clinical dose range that extends up to 750mg/day (up to 800 mg/day in the USA and Canada).[21,22] Although administration is usually twice-daily,[23] a pilot study in patients with schizophrenia or schizoaffective disorder found similar levels of clinical efficacy in patients administered quetiapine on a once-or twice-daily basis.[24] Moreover, our clinical experience has suggested that a more rapid initiation schedule than that described in current prescribing information (300 mg/day by Day 4; 300-400 mg/day by Day 4 in the USA and Canada),[21] with a higher target dose, may be more appropriate in hospitalised adult and adolescent patients with an acute exacerbation of schizophrenia in order to achieve rapid control of symptoms. Some evidence supporting this assertion can be found in a poster that described a randomised, multicentre, double-blind, pilot study of hospitalised patients with acute schizophrenia who had their doses of quetiapine (twice-daily oral administration) titrated to 400 mg by Days 2, 3 or 5, without any increased risk of adverse events compared with classic initiation.[25] The overall frequency of adverse events was similar among the three initiation groups and somnolence was experienced by fewer than 15% of patients in each initiation group; indeed, the lowest incidence of somnolence (8%) was observed in the group of patients using the fastest initiation scheme (400 mg by Day 2). Throughout the study, patient vital signs and laboratory values were similar among the three initiation groups. The somnolence observed in these quetiapine-treated patients is likely to be a result of histamine H1 antagonism to which tolerance usually develops over time. Experience has shown that somnolence with quetiapine is not dose-related, most cases are mild and transient (usually dissipating after approximately 2 weeks), and that the incidence of somnolence does not increase over time. These data support our clinical experience of initiating quetiapine at 200mg/day in the inpatient setting and increasing the dose in increments of 200 mg/day."
Dosing of quetiapine and Clinical Global Improvement scores in a small study of patients with schizophrenia, schizoaffective disorder or other psychotic disorders.
Managing Acute Exacerbations of Schizophrenia: Focus on: Influence of Tolerability on Treatment Selection
Dosing of quetiapine and Clinical Global Improvement scores in a small study of patients with schizophrenia, schizoaffective disorder or other psychotic disorders.
Managing Acute Exacerbations of Schizophrenia: Focus on: Influence of Tolerability on Treatment Selection
Рецепторный профиль анитипсихотиков второго поколения и галоперидола
Receptor Binding Profile of Aripiprazole and Reference Antipsychoticsa
| Receptor | Aripiprazole | Olanzapine | Risperidone | Quetiapine | Ziprasidone | Clozapine | Haloperidol |
|---|---|---|---|---|---|---|---|
| D1 | 265b | 31 | 430 | 455 | 525 | 85 | 210 |
| D2 | 0.34b | 11 | 4 | 160 | 5 | 126 | 0.7 |
| D3 | 0.8b | 49 | 10 | 340 | 7 | 473 | 2 |
| D4 | 44b | 27 | 9 | 1600 | 32 | 35 | 3 |
| 5-HT1A | 1.7b | >10,000 | 210 | 2800 | 3 | 875 | 1100 |
| 5-HT2A | 3.4b | 4 | 0.5 | 295 | 0.4 | 16 | 45 |
| 5-HT2C | 15b | 23 | 25 | 1500 | 1 | 16 | >10,000 |
| a1 | 57 | 19 | 0.7 | 7 | 11 | 7 | 6 |
| H1 | 61b | 7 | 15 | 11 | 50 | 6 | 440 |
| M1 | >10,000 | 1.9 | >10,000 | 120 | >1000 | 1.9 | >150 |
Aripiprazole in the Treatment of Schizophrenia
Подписаться на:
Сообщения (Atom)