OBJECTIVE: Despite the clinical observation that antipsychotics can produce negative symptoms, no previous controlled study, to our knowledge, has evaluated this action in healthy subjects. The present study assessed observer-rated and self-rated negative symptoms produced by conventional and second-generation antipsychotics in healthy volunteers. METHOD: The authors used a double-blind, placebo-controlled trial of single doses of haloperidol (5 mg) and risperidone (2.5 mg) in normal subjects. Thirty-two subjects were administered haloperidol, risperidone, and placebo in a random order. Motor variables and observer-rated negative symptoms were assessed after 3–4 hours and subjective negative symptoms and drowsiness after 24 hours. RESULTS: Neither of the active drugs caused significant motor extrapyramidal symptoms after administration. Haloperidol caused significantly more negative signs and symptoms than placebo on the Scale for the Assessment of Negative Symptoms (SANS) and two self-rated negative symptom scales: the Subjective Deficit Syndrome Scale total score and an analog scale that evaluates subjective negative symptoms. Risperidone caused significantly more negative signs and symptoms than placebo on the Brief Psychiatric Rating Scale (BPRS), the SANS, the Subjective Deficit Syndrome Scale total score, and the analog scale for subjective negative symptoms. After control for drowsiness, risperidone but not haloperidol produced more negative symptoms than placebo on the BPRS and the SANS. Significance was lost for the subjective negative symptoms with both drugs. CONCLUSIONS: Single doses of both haloperidol and risperidone produce negative symptoms in normal individuals. Drowsiness may be an important confounding factor in the assessment of negative symptoms in antipsychotic trials.
Показаны сообщения с ярлыком галоперидол. Показать все сообщения
Показаны сообщения с ярлыком галоперидол. Показать все сообщения
четверг, 26 января 2012 г.
Исследование вторичной негативной симптоматики при приёме антипсихотиков
пятница, 14 января 2011 г.
Эффективность атипичных антипсихотиков при делирии
Haloperidol is the mainstay of delirium treatment.8 Compared with atypical antipsychotics in delirium treatment, haloperidol doses < 3.5 mg/d have not been associated with an increase in extrapyramidal symptoms (EPS).9
Although not devoid of side effects, atypical antipsychotics are an alternative to haloperidol.8,10 This article briefly summarizes the current evidence on the use of atypicals for treating delirium.
Evidence for antipsychotics
Haloperidol has been the antipsychotic of choice for treating delirium symptoms. It is recommended by the Society of Critical Care Medicine7 and is regarded as safe, cost-effective, and efficacious for delirium5 despite a risk of dose-related EPS and potential cardiac conduction alterations.5,14
Risperidone is not indicated for treating delirium but is one of the most extensively studied atypical antipsychotic alternatives to haloperidol. Evidence consisting primarily of case reports has illustrated the potential efficacy of risperidone in treating delirium (Table 2).10,15-19
Clinical Point
In a small double-blind, randomized trial, risperidone was effective but not significantly more so than low-dose haloperidol
In 2004, Parellada et al17 observed significant mean improvements in all measures (Delirium Rating Scale [DRS], Mini-Mental State Exam [MMSE], positive subscale of the Positive and Negative Syndrome Scale [PANSS-P], and Clinical Global Impressions scale [CGI]) in 64 delirium patients treated with risperidone. In a 2004 double-blind trial of 28 delirium patients randomly assigned to risperidone or haloperidol, risperidone was effective but not significantly more efficacious than low-dose haloperidol for acute delirium treatment.18
Advantages of using risperidone include its lack of anticholinergic effects. Potential side effects include dose-related EPS and weight gain, which were observed in patients with schizophrenia and other psychotic disorders and dementia-related behavioral disorders.20,21
Olanzapine. Much like risperidone, olanzapine’s use in delirium is relatively well described in the literature (Table 3).22-24 In a randomized, placebo-controlled study comparing olanzapine with haloperidol, 175 patients were treated for 7 days with olanzapine, haloperidol, or placebo. Olanzapine and haloperidol showed significantly greater DRS score improvement than placebo.24 There was no difference between olanzapine and haloperidol outcomes; however, olanzapine showed significant improvement by days 2 and 3 compared with haloperidol. Haloperidol was associated with a significantly higher rate of dystonia compared with olanzapine.
Olanzapine carries a risk of anticholinergic effects. This can be a drawback, especially in patients such as Ms. B whose delirium has an anticholinergic component. Olanzapine is available in an IM formulation, which can be an advantage when addressing agitation and medical comorbidities of delirium.
Quetiapine. Case reports have suggested quetiapine is effective for delirium (Table 4).10,25-27 In a prospective, open-label trial, Sasaki et al26 treated 12 delirium patients with a single bedtime dose of quetiapine. All patients achieved remission within several days of beginning quetiapine, and the drug was well tolerated with no detected EPS or excessive sedation.
Clinical Point
Quetiapine reduced delirium duration and agitation in a small double-blind randomized trial of adult ICU patients
In 2010 Devlin et al27 reported on the efficacy and safety of quetiapine in a prospective double-blind, placebo-controlled study of 36 adult ICU patients. Compared with those receiving placebo, patients taking quetiapine had a statistically significant shorter time to first resolution of delirium, reduced duration of delirium, and less agitation as measured by the Sedation-Agitation Scale. Mortality, ICU length of stay, and incidence of QTc prolongation did not differ, but patients treated with quetiapine were more likely to be discharged home or to rehabilitation and to have more somnolence. Quetiapine’s side effect profile includes a low occurrence of EPS, sedation, and dose-related anticholinergic effects.25
Ziprasidone. The literature on ziprasidone for delirium so far is limited to a few anecdotal case reports (Table 5).28-31 In 2002, Leso and Schwartz28 successfully used ziprasidone to treat delirium in a patient with human immunodeficiency virus and cryptococcal meningitis. Ziprasidone was chosen for its lack of sedating effects and low EPS risk. The patient experienced significant clearing of his delirium and lowering of his DRS score. Ziprasidone eventually was discontinued because a fluctuating QTc interval associated with comorbid electrolyte imbalances—a potential drawback to ziprasidone.
In the case of Ms. B, ziprasidone appeared to be efficacious; however, improvement in her medical condition, rather than ziprasidone treatment, is the most likely explanation for the resolution of her delirium symptoms.
Aripiprazole. Alao et al30 reported on 2 delirium patients treated with 30 mg and 15 mg aripiprazole; improvement was monitored using the MMSE and DRS (Table 5).28-31 In both cases, confusion, disorientation, and agitation improved within 7 days of treatment. In the first case, the patient’s MMSE score improved from 5 to 28 and his DRS score decreased from 28 to 6. The second patient’s MMSE score improved from 7 to 27 and her DRS score went from 18 to 6.
Straker et al31 reported on 14 delirium patients treated with aripiprazole. Twelve patients had a ≥50% reduction in DRS, Revised-98 scores, and 13 showed improvement on CGI scores. The rate of adverse side effects was low. Three patients had prolonged QTc interval, but no patients developed arrhythmia or discontinued aripiprazole.
Atypical antipsychotics for delirium: A reasonable alternative to haloperidol?
понедельник, 22 ноября 2010 г.
Сравнение атипичных антипсихотиков



For example, when switching from a tightly binding anticholinergic or antihistaminergic medication (eg, olanzapine, quetiapine, clozapine) to one with less anticholinergic or antihistaminergic affinity (eg, aripiprazole, risperidone, ziprasidone), often transient rebound anxiety, insomnia, agitation and restlessness can occur. In addition, when switching from a tighter D2 binding agent to a looser-binding agent (eg, from risperidone to clozapine or quetiapine) or, particularly, to a partial dopamine agonist (eg aripiprazole) dopamine rebound symptoms, such as often transient worsening of psychosis, mania or aggression/agitation, can occur. A pharmacokinetic dopamine rebound may also occur when switching from a short half-life antipsychotic to a longer half-life antipsychotic (Table 1).4
The abrupt switch has the greatest potential for rebound and withdrawal phenomena. Even the conventional cross-titration can lead to problems when the pre-switch antipsychotic has a shorter half life and/or blocks more tightly cholinergic, histaminergic or dopaminergic receptors than the post-switch antipsychotic. Rebound phenomena can be minimized by avoiding abrupt or fast switching when the pre- and post-switch receptor affinities and/or half-lives differ considerably. Instead, an overlapping or “plateau” switch should be used. This consists of decreasing the pre-switch antipsychotic slowly (eg, 25–50% every 5 half-lives) and only after the post-switch antipsychotic has reached steady state (ie, ≤5 half lives on target dose). Adding calming medications during the switch period, such as benzodiazepines, antihistamines or sleep aides, can also minimize rebound phenomena.
A number of non-antipsychotic augmentation strategies have also been tested in schizophrenia patients with insufficient response to antipsychotic monotherapy. Of these, lithium,16 carbamazepine,17 and beta blockers18 were not superior to placebo when added to an antipsychotic. Similarly, benzodiazepine19 and valproate augmentation20 also did not show long-term superiority compared to placebo, although both agents might speed up the initial response. Although two large-scale studies showed no superiority of lamotrigine augmentation of antipsychotics compared to placebo,21 a meta-analysis demonstrated significant superiority regarding global ratings of psychopathology, positive and negative symptom change, as well as study-defined response when outcomes of patients were combined in whom lamotrigine was added to clozapine.22 This, however, has not been verified in a prospective study.
ECT augmentation has also been shown to be superior, both for acute efficacy and in maintenance treatment, when added to antipsychotic monotherapy in patients who have failed antipsychotic monotherapy.23
One meta-analysis suggested that augmentation of antipsychotics with antidepressants may be more helpful than placebo for schizophrenia patients with predominantly negative symptoms.24 Larger, validating studies are needed, however, and specific effects on negative symptoms need to be distinguished from proven effects of antidepressants on depressive symptoms in schizophrenia patients.25
Practical Dosing Strategies in the Treatment of Schizophrenia: Part 2 - Switching and Combining Antipsychotics
Монотерапия рисперидоном в сравнении с терапией комбинацией низких доз рисперидона и галоперидола
Monotherapy is recommended for schizophrenia treatment, but the risk-benefit issue of antipsychotic drug combination (except for clozapine) remains unclear. Risperidone, an atypical antipsychotic drug, has a lower incidence of extrapyramidal syndrome but higher risks of prolactinemia and metabolic syndrome than haloperidol, a typical agent. This study compared efficacy and safety of risperidone monotherapy versus low-dose risperidone plus low-dose haloperidol in schizophrenia. In this 6-week, double-blind study, patients were randomized to the combination group (2-mg/d risperidone plus 2-mg/d haloperidol, n = 46) or the monotherapy group (4-mg/d risperidone, n = 42). Efficacy assessments included Clinical Global Impression-Severity, Positive and Negative Syndrome Scale and subscales, Calgary Depression Scale, Global Assessment of Functioning, and Medical Outcomes Study Short-Form 36. Safety was rigorously monitored. Response was defined as 30% reduction in the Positive and Negative Syndrome Scale total score. The 2 treatment groups were similar in (1) demographic and clinical characteristics at baseline, (2) response rate, and (3) improvement in various psychopathological measures and quality of life at end point. The monotherapy group had a higher increase in prolactin levels (P = 0.04) and Simpson-Angus Scale scores (P = 0.04) and a higher percentage of biperiden use (P = 0.045). There were no significant between-group difference in changes in weight, vital signs, corrected QT interval, liver/renal function, fasting glucose level, and lipid profiles. The findings suggest that risperidone monotherapy may yield higher prolactin levels than a combination of low-dose risperidone plus low-dose haloperidol. The 2 treatment groups are similar in efficacy, life quality, and other safety profiles. Future long-term studies are warranted.
A randomized, double-blind comparison of risperidone versus low-dose risperidone plus low-dose haloperidol in treating schizophrenia.
Потенциирование клозапина: сульпирид, амисульприд, ламотриджин
A frequent treatment strategy for clozapine-resistant patients with schizophrenia is the use of specific augmentors that are suitable for adjunctive therapy. Clozapine is a polyvalent drug but it lacks high-potency dopamine receptor blockade (Kerwin & Osborne, 2000). Therefore, there has been interest in using as augmentors substituted benzamides with highly selective dopamine receptor blocking profiles (Kerwin, 2000). Augmentation strategies incorporating sulpiride are well documented. The authors of one study of sulpiride augmentation in 28 patients partially responsive to clozapine (Shiloh et al, 1997) noted a mean reduction of about 40–50% in various clinical response scores (Brief Psychiatric Rating Scale and Scale for the Assessment of Positive Symptoms).
Several groups have been interested in mimicking this study with amisulpride, a relative of sulpiride that is even more selective at the dopamine D2 receptor. A case series by Zink et al(2004) showed improvement in previously treatment-resistant symptoms following a combined treatment strategy of clozapine and amisulpride. In addition, our group performed an open trial of amisulpride augmentation in a long-term (52 weeks) study. Significant improvement was observed in half of the patients, with no additional side-effects. Moreover, this study monitored plasma levels to determine whether this was a pharmacokinetic interaction. Clozapine levels did not change throughout the duration of the trial, suggesting a pharmacodynamic interaction (Munro et al, 2004).
Augmentation with anti-epileptics
A glutamate hyperfunction hypothesis of schizophrenia has generated interest in the role of glutamate release inhibitors as clozapine augmentors. In a study of 26 treatment-resistant patients receiving lamotrigine (17) or topirimate (9) in addition to their existing antipsychotic treatment (a variety of antipsychotics), a significant improvement was observed when lamotrigine was added to risperidone, haloperidol, olanzapine or flupenthixol. However, no significant effect was observed in patients receiving topirimate augmentation in addition to clozapine, olanzapine, haloperidol or flupenthixol (Dursun & Deakin, 2001). The therapeutic effects of lamotrigine augmentation were also assessed in a rigorous randomised placebo-controlled cross-over study of 34 clozapine-resistant patients (Tiihonen et al, 2003). In this 14-week study, lamotrigine treatment significantly improved positive symptoms and general psychopathological symptoms, but had no effect on negative symptoms. The authors suggested that this was the first time a non-dopamine antagonist had proven efficacy in schizophrenia, giving further credence to the hyperglutamate neurotransmission hypothesis for the generation of positive symptoms in the disorder.
Management of clozapine-resistant schizophrenia
вторник, 19 октября 2010 г.
Normally, after eating, your body uses carbohydrate as the main energy source. After a long time hungry, it switches to fat. Zyprexa made the body use fat all the time
SUMMARY: A class effect, to varying degrees; and eating less may not help.
1. Food intake was the same between controls and Zyprexaers. You get these effects even if you eat the same.
2. This effect is shared by other atypicals, in a predictable fashion:
In the fed state, Zyprexa and Clozaril do a massive conversion to fat utilization, Risperdal a medium, and sulpiride minimal covnersion.
In the fasting state:
Geodon has a lesser effect than Zyprexa, and appears to normalize; Abilify and Haldol seem close to normal.
3. These effects are consistent with Lilly's own studies that the majority of weight gain happens in the first month, and not suddenly after a year of use.
4. There is still a hunger component to weight gain that is separate from the metabolic effect. Some drugs will make you hungry, change your metabolism, or some mixture of the two. Hunger appears to be a H1 mediated process (Seroquel, Zyprexa, Clozaril, Remeron, Paxil>Prozac, etc.)
5. The immediate clinical consequence of this information is probably (paradoxically) to tell the patients to eat less sugar.
Unless you dramatically cut fat out of your diet, the body will still churn through what fat you do eat at the expense of carbohydrate. Better, and easier, to reduce the carb load that lingers in your body (and likely ultimately gets stored.)
Why Zyprexa (And Other Atypical Antipsychotics) Make You Fat
вторник, 21 сентября 2010 г.
Смертность пациентов с деменцией получающих антипсихотические препараты
They found that no antipsychotic was associated with greater long-term mortality, but that haloperidol, olanzapine, and risperidone, but not quetiapine, were associated with a short-term increase in mortality during the first 30 days of prescribing.
Are Commonly Prescribed Antipsychotics Associated With Greater Mortality in Patients With Dementia?
вторник, 29 июня 2010 г.
пятница, 18 июня 2010 г.
вторник, 15 июня 2010 г.
Селективность антипсихотиков in vitro и ex vivo
In a recent human [11C]-(+)-PHNO positron emission tomography study, olanzapine, clozapine, and risperidone occupied D2 receptors in striatum (STR), but, despite their similar in vitro D2 and D3 affinities, failed to occupy D3 receptors in globus pallidus. This study had two aims: (1) to characterize the regional D2/D3 pharmacology of in vitro and ex vivo [3H]-(+)-PHNO binding sites in rat brain and (2) to compare, using [3H]-(+)-PHNO autoradiography, the ex vivo and in vitro pharmacology of olanzapine, clozapine, risperidone, and haloperidol. Using the D3-selective drug SB277011, we found that ex vivo and in vitro [3H]-(+)-PHNO binding in STR is exclusively due to D2, whereas that in cerebellar lobes 9 and 10 is exclusively due to D3. Surprisingly, the D3 contribution to [3H]-(+)-PHNO binding in the islands of Calleja, ventral pallidum, substantia nigra, and nucleus accumbens was greater ex vivo than in vitro. Ex vivo, systemically administered olanzapine, risperidone, and haloperidol, at doses occupying ~80% D2, did not occupy D3 receptors. Clozapine, which also occupied ~80% of D2 receptors ex vivo, occupied a smaller percentage of D3 receptors than predicted by its in vitro pharmacology. Across brain regions, ex vivo occupancy by antipsychotics was inversely related to the D3 contribution to [3H]-(+)-PHNO binding. In contrast, in vitro occupancy was similar across brain regions, independent of the regional D3 contribution. These data indicate that at clinically relevant doses, olanzapine, clozapine, risperidone, and haloperidol are D2-selective ex vivo. This unforeseen finding suggests that their clinical effects cannot be attributed to D3 receptor blockade.
The Antipsychotics Olanzapine, Risperidone, Clozapine, and Haloperidol Are D2-Selective Ex Vivo but Not In Vitro
четверг, 3 июня 2010 г.
Рабочие дозы антипсихотиков
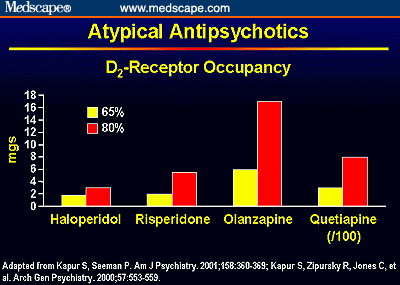
This bar chart illustrates the dose of antipsychotic medications needed to occupy D2-dopamine receptors at 65% (at which point the antipsychotic effect begins) and 80% (at which point EPS begins). The wider dosing margin between these occupancy levels with newer antipsychotics explains the lower EPS liability of these drugs. The quetiapine dose is divided by 100 to fit on the chart; note that it is unknown if quetiapine occupies 80% of D2-dopamine receptors at any dose, hence the low EPS liability of this compound.
Long-term Tolerability of Second-Generation Antipsychotics in Bipolar Disorder
четверг, 20 мая 2010 г.
Рецепторный профиль анитипсихотиков второго поколения и галоперидола
Receptor Binding Profile of Aripiprazole and Reference Antipsychoticsa
| Receptor | Aripiprazole | Olanzapine | Risperidone | Quetiapine | Ziprasidone | Clozapine | Haloperidol |
|---|---|---|---|---|---|---|---|
| D1 | 265b | 31 | 430 | 455 | 525 | 85 | 210 |
| D2 | 0.34b | 11 | 4 | 160 | 5 | 126 | 0.7 |
| D3 | 0.8b | 49 | 10 | 340 | 7 | 473 | 2 |
| D4 | 44b | 27 | 9 | 1600 | 32 | 35 | 3 |
| 5-HT1A | 1.7b | >10,000 | 210 | 2800 | 3 | 875 | 1100 |
| 5-HT2A | 3.4b | 4 | 0.5 | 295 | 0.4 | 16 | 45 |
| 5-HT2C | 15b | 23 | 25 | 1500 | 1 | 16 | >10,000 |
| a1 | 57 | 19 | 0.7 | 7 | 11 | 7 | 6 |
| H1 | 61b | 7 | 15 | 11 | 50 | 6 | 440 |
| M1 | >10,000 | 1.9 | >10,000 | 120 | >1000 | 1.9 | >150 |
Aripiprazole in the Treatment of Schizophrenia
среда, 12 мая 2010 г.
Атипичные антипсихотики: сон, седация и эффективность
"In general, the high-milligram, low-potency antipsychotics, such as chlorpromazine and mesoridazine, produce more sedation than the low-milligram, high-potency antipsychotics such as haloperidol and fluphenazine (Table 1).6 This principle tends to hold true for the atypical antipsychotics as well. For example, the high-potency, low-dose atypical antipsychotic risperidone is less sedating than the lower-potency, high-dose atypical antipsychotics quetiapine and clozapine. However, dose does not always determine sedation. Olanzapine, which has a common dose range of 15 to 30 mg/day, is more sedating than ziprasidone, which has a common dose range of 80 to 160 mg/day. Studies have indicated that sedation may also be related to the affinity of the medication for the histamine H1 receptors. The antipsychotics vary in their ability to block these receptors.4,7 A study by Richelson and Souder7 of the binding profiles of antipsychotic medications found that olanzapine has the highest affinity for the histamine H1 receptors, followed by clozapine (Figure 1Figure 1.). This may explain why olanzapine has a relatively large sedative effect even though it is a high-potency medication. Of the antipsychotics studied, haloperidol had the lowest affinity for the histamine H1 receptors. Quetiapine and risperidone had the lowest affinity of the atypical antipsychotics. Although both dosage and affinity for histamine H1 receptors play a part in the sedative effect of a medication, what ultimately determines sedative effect is the amount of the drug reaching the histamine H1 receptors in the central nervous system. For example, quetiapine, which has little affinity for the histamine H1 receptors, is a less potent antipsychotic medication and requires many more milligrams to be effective than do higher-potency medications such as risperidone and ziprasidone. Because of this, quetiapine has a greater sedative effect on patients in clinical use than do risperidone and ziprasidone."
"If sedation is bothersome to patients taking antipsychotic medications, physicians can take steps to minimize it. According to the 1999 Expert Consensus Guidelines on the treatment of schizophrenia,15 physicians should consider eliminating other sedating agents from the patient's list of medications. This includes antidepressants, such as the tricyclics and mirtazapine, and mood-stabilizing medications such as valproic acid. Instructing the patient to take his or her medication at bedtime can also reduce daytime sedation. If the entire dose cannot be given at bedtime, then the majority of the dose should be taken at night. If necessary, the physician should consider reducing the dose of the antipsychotic medication, but this should be done slowly and cautiously. The physician could also consider switching the patient to a less sedating antipsychotic. Also, the physician might consider checking the patient for hypothyroidism, which can cause individuals to feel sedated. If these efforts do not work, caffeine or bupropion might help the patient feel more alert. Many patients taking antipsychotic medications drink several cups of coffee every morning to feel less sedated.
The 1999 guidelines recommended prescribing stimulants for patients who were persistently sedated, but this has become highly controversial. Generally, I do not recommend prescribing stimulants for psychotic patients because sedation can usually be controlled using other means and the physician may be held liable for the patient's actions while medicated with stimulants.
A medication that has recently emerged as an option to treat drug-induced sedation is modafinil. The treatment mechanism of modafinil is unknown, and although it is a schedule IV controlled substance, it is not a stimulant. It has been used successfully in clinical settings to combat sedation, but there is concern that it may worsen psychotic symptoms. Modafinil was reported to have exacerbated psychosis in 1 patient who was taking a dose of 200 mg 4 times daily,16 but no adverse effects were reported in 3 patients who were taking 200 mg/day along with antipsychotic medications.17"
Atypical Antipsychotics: Sleep, Sedation, and Efficacy
"If sedation is bothersome to patients taking antipsychotic medications, physicians can take steps to minimize it. According to the 1999 Expert Consensus Guidelines on the treatment of schizophrenia,15 physicians should consider eliminating other sedating agents from the patient's list of medications. This includes antidepressants, such as the tricyclics and mirtazapine, and mood-stabilizing medications such as valproic acid. Instructing the patient to take his or her medication at bedtime can also reduce daytime sedation. If the entire dose cannot be given at bedtime, then the majority of the dose should be taken at night. If necessary, the physician should consider reducing the dose of the antipsychotic medication, but this should be done slowly and cautiously. The physician could also consider switching the patient to a less sedating antipsychotic. Also, the physician might consider checking the patient for hypothyroidism, which can cause individuals to feel sedated. If these efforts do not work, caffeine or bupropion might help the patient feel more alert. Many patients taking antipsychotic medications drink several cups of coffee every morning to feel less sedated.
The 1999 guidelines recommended prescribing stimulants for patients who were persistently sedated, but this has become highly controversial. Generally, I do not recommend prescribing stimulants for psychotic patients because sedation can usually be controlled using other means and the physician may be held liable for the patient's actions while medicated with stimulants.
A medication that has recently emerged as an option to treat drug-induced sedation is modafinil. The treatment mechanism of modafinil is unknown, and although it is a schedule IV controlled substance, it is not a stimulant. It has been used successfully in clinical settings to combat sedation, but there is concern that it may worsen psychotic symptoms. Modafinil was reported to have exacerbated psychosis in 1 patient who was taking a dose of 200 mg 4 times daily,16 but no adverse effects were reported in 3 patients who were taking 200 mg/day along with antipsychotic medications.17"
Atypical Antipsychotics: Sleep, Sedation, and Efficacy
вторник, 2 марта 2010 г.
Психотическая симптоматика после злоупотребления энергетическими напитками у больного шизофренией
Caffeine functions in the central nervous system as a competitive antagonist of adenosine receptors, A1 and A2A, and alters neurotransmitter release, including dopamine and glutamate. This dopamine release in the striatum may underlie caffeine's reinforcing properties, and the modulation of the mesolimbic dopamine pathway may be related to its psychotomimetic effect (1). Exaggerated effects may be seen in patients with schizophrenia using high-dose caffeine (2). The energy drink the patient in the present case consumed contained 160 mg of caffeine per can. The patient weighed 67 kg and therefore consumed approximately 20 mg/kg per day of caffeine. Psychosis has been reported at doses near 10 mg/kg per day (3), which is well below the known toxic dosage (150–200 mg/kg) but above the average intake by the numerous schizophrenia outpatients who use caffeine (1.8–4.1 mg/kg per day [depending on smoking status]) (4).
Psychosis Following Excessive Ingestion of Energy Drinks in a Patient With Schizophrenia
Psychosis Following Excessive Ingestion of Energy Drinks in a Patient With Schizophrenia
пятница, 19 февраля 2010 г.
Атипичность атипичных антипсихотиков
The main reason for the development of atypical antipsychotics was to provide an antipsychotic treatment option that was free of EPS and, further, to reduce the risk of tardive dyskinesia. Although many agents now claim to be atypical, the data presented suggest that only clozapine and quetiapine are true atypical antipsychotics. Of the available agents, clozapine and quetiapine show the lowest affinity for and the most rapid release from D2 receptors.3 As a result, clozapine and quetiapine have been shown to have an incidence of EPS that was no different from placebo across the full dosage range.4
Promotion of risperidone, olanzapine, ziprasidone, and possibly aripiprazole has emphasized the atypicality of these agents; however, based on the data presented here, one should question whether these agents are truly atypical antipsychotics. Both risperidone and olanzapine have demonstrated EPS levels similar to placebo at low doses; at higher doses, however, EPS incidence for both agents becomes greater than with placebo.4 Data for ziprasidone are limited; however, ziprasidone appears to have an EPS risk similar to that of risperidone and olanzapine.56,57 Ari-piprazole has been associated with akathisia; however, further study and experience with this drug are needed before definitive conclusions can be drawn.
Atypicality of Atypical Antipsychotics
Promotion of risperidone, olanzapine, ziprasidone, and possibly aripiprazole has emphasized the atypicality of these agents; however, based on the data presented here, one should question whether these agents are truly atypical antipsychotics. Both risperidone and olanzapine have demonstrated EPS levels similar to placebo at low doses; at higher doses, however, EPS incidence for both agents becomes greater than with placebo.4 Data for ziprasidone are limited; however, ziprasidone appears to have an EPS risk similar to that of risperidone and olanzapine.56,57 Ari-piprazole has been associated with akathisia; however, further study and experience with this drug are needed before definitive conclusions can be drawn.
Atypicality of Atypical Antipsychotics
понедельник, 7 декабря 2009 г.
воскресенье, 11 октября 2009 г.
Нейролептики при деменции
После рекомендации воздерживаться от употребления атипичных нейролептиков из-за повышенного риска инсультов, Trifiro отметил убедительный рост количества назначений классических антипсихотиков. Но эти препараты несут такой же высокий (зависимый от дозы) риск инсульта, что и атипичные антипсихотики, по сравнению с пациентами, которые не принимали никаких психотропных препаратов. В некоторых подгруппах классических нейролептиков риск намного выше, в частности, 5,8 для фенотиазинов и 3,6 для бутирофенонов (например, галоперидола). Trifiro призывает максимально ограничить использование обоих классов лекарств. ''Употреблять их только при необходимости, в самых низких дозах и в течение максимально короткого времени''. Trifiro не отмечает при использовании классических антипсихотиков повышенного риска (в т.ч. фатальной) пневмонии, по сравнению с атипичными антипсихотиками. Вместе с тем, он ожидал повышенных рисков в связи с действием на экстрапирамидную систему, что вызывает акинезию и впоследствии может вызвать аспирационную пневмонию. Но, как оказалось, летальные случаи от воспаления легких чаще встречались при использовании атипичных антипсихотиков. Вероятность пневмонии возрастает в связи с аффинитетом антипсихотиков к гистаминергическим рецепторам Н1. Именно поэтому у фенотиазинов самый высокий сравнительный риск 4,3 – по сравнению с пациентами, которые не принимают психотропных препаратов. Trifiro объясняет это тем, что гистаминовые рецепторы участвую в седации, а седация может создавать проблемы с глотанием и впоследствии аспирационную пневмонию.
МНИИП
МНИИП
Подписаться на:
Сообщения (Atom)
