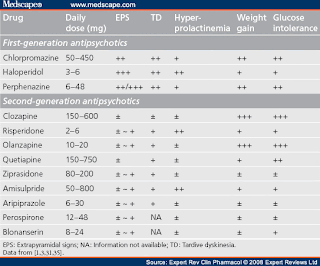
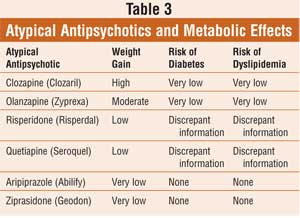
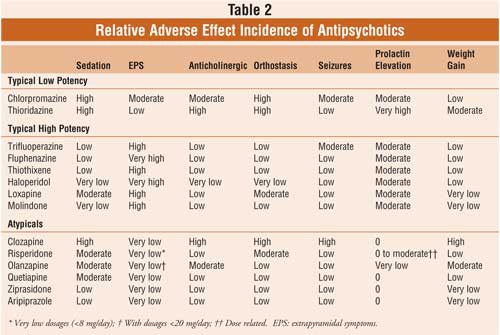
Topline results from a phase 2 study show that treatment with a novel oral antipsychotic was as effective as treatment with the antipsychotic olanzapine (multiple brands) with far less weight gain in patients with schizophrenia, the company developing the drug announced today.Positive Topline Phase 2 Results for Novel Schizophrenia Drug
The drug currently known as ALKS 3831, from Alkermes, combines samidorphan, a novel, potent mu-opioid antagonist, with olanzapine.
Показаны сообщения с ярлыком масса тела. Показать все сообщения
Показаны сообщения с ярлыком масса тела. Показать все сообщения
пятница, 23 января 2015 г.
Самидорфан и оланзапин
четверг, 1 ноября 2012 г.
Антидепрессивные свойства оланзапина
Randomised, double-blind, placebo-controlled study of olanzapine in patients with bipolar I depression*BackgroundAtypical antipsychotics are widely used in bipolar mania. However, the efficacy of atypical antipsychotics in bipolar depression has not been comprehensively explored.AimsTo evaluate olanzapine monotherapy in patients with bipolar depression.MethodPatients with bipolar depression received olanzapine (5–20 mg/day, n = 343) or placebo (n = 171) for 6 weeks. The primary outcome was change from baseline to end-point in Montgomery–Åsberg Depression Rating Scale (MADRS) total score. Secondary outcomes included: Clinical Global Impression – Bipolar Version (CGI-BP) scale, 17-item Hamilton Rating Scale for Depression (HRSD-17) and Young Mania Rating Scale (YMRS) scores, and the rate of response (50% reduction in MADRS at end-point), recovery (MADRS 12 for 4 weeks plus treatment completion) and remission (MADRS 8). The trial was registered with ClinicalTrials.gov (NCT00510146).ResultsOlanzapine demonstrated: significantly greater (P < 0.04) improvements on MADRS (least-squares mean change –13.82 v. –11.67), HRSD-17 and YMRS total scores and all CGI-BP subscale scores v. placebo; significantly (P⩽0.05) more response and remission, but not recovery; significantly (P < 0.01) greater mean increases in weight, fasting cholesterol and triglycerides; and significantly more (P < 0.001) patients gained ⩾7% body weight.ConclusionsOlanzapine monotherapy appears to be efficacious in bipolar depression. Additional long-term studies are warranted to confirm these results. Safety findings were consistent with the known safety profile of olanzapine.
пятница, 3 августа 2012 г.
Бетагистин как корректор увеличения массы тела при приёме оланзапина
Olanzapine is effective at treating multiple domains of schizophrenia symptoms. However, it induces serious metabolic side effects. Antipsychotic drug’s antagonistic affinity to histamine H1 receptors has been identified as a main contributor for weight gain/obesity side effects. This study therefore investigated whether a combined treatment of betahistine (a H1 receptor agonist and H3 receptor antagonist) could reduce the body weight/obesity induced by olanzapine. Female Sprague Dawley rats were treated orally with olanzapine (1 mg/kg, t.i.d.) and/or betahistine (2.67 mg/kg, t.i.d.), or vehicle for two weeks. Rats treated with olanzapine exhibited significant body weight gain and increased food intake. Co-treatment of olanzapine with betahistine significantly prevented (-45%) weight gain and reduced feeding efficiency compared to sole olanzapine treatment. Betahistine treatment alone had no effect on weight gain and food intake. Olanzapine reduced locomotor activity, but not betahistine. These findings demonstrate that olanzapine-induced body weight gain can partially be reduced by co-treatment with betahistine. Betahistine has H3 receptor antagonistic effects to increase histamine release, which may augment its direct agonistic effects on H1 receptors. These findings have important implications for clinical trials using betahistine to control antipsychotic-induced obesity side effects.Reducing olanzapine-induced weight gain side effect by using betahistine: a study in the rat model
среда, 18 апреля 2012 г.
Пиколинат хрома, депрессия и контроль массы тела
A Duke University study validated that adding chromium supplements to diet significantly improved symptoms of atypical depression, and patients’ tendency to overeat. In the study, 75 people with atypical depression, most of whom were overweight or obese, received either 600 mcg per day of chromium (as chromium picolinate) or a placebo for eight weeks. The proportion of people who improved by at least 50% (responders) was greater in the chromium group than in the placebo group (54% versus 36%), although this difference was not statistically significant. However, significant differences were seen when the analysis was restricted to those people who reported that they had severe carbohydrate craving (a possible indicator of abnormal glucose metabolism). In that subset, the proportion of responders was 65% in the chromium group and only 33% in the placebo group, a statistically significant difference. No significant side effects were observed in people taking chromium.Chromium, Depression, and Weight Management
вторник, 31 января 2012 г.
Генетические маркеры метаболических осложнений при приёме антипсихотиков второго поколения
Using DNA analysis, the researchers found that 8% of all study participants had the MTHFR C677T gene variant. Those with the variant who also used SGAs were significantly more likely to have metabolic syndrome than the children receiving SGAs but without the variant (OR, 5.75; P < .05).
The children with the gene variant also had higher diastolic blood pressure (P = .005) and higher fasting plasma glucose (P < .05).
"This is the first report of an underlying biological factor predisposing children to complications associated with SGA medication use. However, we need to do more research before this can be translated into clinical practice," said Dr. Panagiotopoulos.
She noted that because it is known that the MTHFR gene is involved in metabolizing the B-vitamin folate, the investigators are currently taking a more detailed dietary history from these children and conducting a study to answer "what's going on?" with the population's nutritional intake.
вторник, 26 апреля 2011 г.
Метаболические расстройства коморбидные с БАР

Chronic stress, which patients experience during both the manic and the depressive phases of bipolar disorder, is associated with increased cortisol levels, lack of cortisol suppression, and changes in hypothalamic-pituitary-adrenal axis responses. This metabolic dysregulation may increase insulin resistance and can lead to hyperglycemia, increased oxidative stress, metabolic syndrome, and atherosclerosis. In addition, patients with bipolar illness have increased activity of the sympathetic nervous system, which may also lead to insulin resistance, metabolic syndrome, and increased risk of sudden cardiac death.2
Depressive syndromes may be neurotoxic. Abnormalities in cellular plasticity, cellular resilience, and intracellular signaling, as well as alterations in the size, shape, and density of neurons and glia, have been found. Studies employing neuroimaging and neuropsychological tests have demonstrated abnormalities in brain morphology and function in patient populations with depressive syndromes and in those with diabetes. Common physiologic mechanisms have been implicated, including insulin-glucose homeostasis, immuno-inflammatory processes, and oxidative stress mechanisms.
Metabolic Comorbidities in Patients With Bipolar Disorder
суббота, 5 марта 2011 г.
пятница, 21 января 2011 г.
Коррекция метаболических побочных эффектов антипсихотиков
This systematic review and meta-analysis included 32 randomized, open and double-blind, placebo-controlled studies (mean duration: 13.1 weeks, range: 6-16 weeks) with a total of 1482 subjects and which tested the following 15 medications: amantadine, dextroamphatamine, d-fenfluramine, famotidine, fluoxetine, fluvoxamine, metformin, nizatidine, orlistat, phenylpropanolamine, reboxetine, rosiglitazone, sibutramine, topiramate, and metformin plus sibutramine).
Five of the agents assessed lead to significantly greater weight loss than placebo. The greatest weight loss was achieved with metformin (N = 7, n = 334, -2.94 kg [CI, -4.89, -0.99]), followed by d-fenfluramine (N = 1, n = 16, -2.60 kg [CI,-5.14, -0.06]), sibutramine (N = 2, n = 55, -2.56 kg [CI, -3.91, -1.22]), topiramate (N = 2, n = 133, -2.52, [CI, -4.87, -0.16]) and reboxetine (N = 2, n = 79, -1.90 kg [CI, -3.07, -0.72]). Nausea rates did not differ between treatment and placebo groups. Results on the secondary outcome measures of waist circumference and weight gain, carbohydrate metabolism and blood lipids as well as sensitivity analysis regarding prevention vs intervention trials were largely heterogeneous. No significant differences between treatment and placebo groups were found for the secondary outcome measures of psychiatric symptoms and adverse events.
The Year in Psychosis and Bipolar Disorder: Treating Antipsychotic-related Metabolic Abnormalities
суббота, 13 ноября 2010 г.
Влияние гипертензии и индекса массы тела на когнитивные функции больных шизофренией
Objective: In recent years there has been a greater appreciation of the elevated prevalence of cardiovascular risk factors in the schizophrenia population and the liability some treatments have for their development. These vascular risk factors are in turn important risk factors in the development of dementia and more subtle cognitive impairments. However, their impact on the cognitive functions of patients with schizophrenia remains underexplored. The authors investigated whether vascular risk factors influence the cognitive impairments of schizophrenia and whether their effects on cognition in schizophrenia are different from those observed in nonpsychiatric comparison subjects.
Method: The authors compared 100 patients with schizophrenia and 53 comparison subjects on cognitive test performance in 2x2 matrices composed of individual vascular risk factors and group (schizophrenia patients and comparison subjects).
Results: Hypertension exerted a significant negative effect on immediate delayed and recognition memory in both groups. Patients with schizophrenia and hypertension were adversely affected in recognition memory, whereas comparison subjects were not. A body mass index above 25 was associated with negative effects on delayed memory in both groups, although the association fell short of statistical significance.
Conclusions: Given that patients with schizophrenia have a higher prevalence of vascular risk factors than the general population and are undertreated for them, treatment of these risk factors may significantly improve cognitive outcome in schizophrenia.
The Effects of Hypertension and Body Mass Index on Cognition in Schizophrenia
вторник, 19 октября 2010 г.
Normally, after eating, your body uses carbohydrate as the main energy source. After a long time hungry, it switches to fat. Zyprexa made the body use fat all the time
SUMMARY: A class effect, to varying degrees; and eating less may not help.
1. Food intake was the same between controls and Zyprexaers. You get these effects even if you eat the same.
2. This effect is shared by other atypicals, in a predictable fashion:
In the fed state, Zyprexa and Clozaril do a massive conversion to fat utilization, Risperdal a medium, and sulpiride minimal covnersion.
In the fasting state:
Geodon has a lesser effect than Zyprexa, and appears to normalize; Abilify and Haldol seem close to normal.
3. These effects are consistent with Lilly's own studies that the majority of weight gain happens in the first month, and not suddenly after a year of use.
4. There is still a hunger component to weight gain that is separate from the metabolic effect. Some drugs will make you hungry, change your metabolism, or some mixture of the two. Hunger appears to be a H1 mediated process (Seroquel, Zyprexa, Clozaril, Remeron, Paxil>Prozac, etc.)
5. The immediate clinical consequence of this information is probably (paradoxically) to tell the patients to eat less sugar.
Unless you dramatically cut fat out of your diet, the body will still churn through what fat you do eat at the expense of carbohydrate. Better, and easier, to reduce the carb load that lingers in your body (and likely ultimately gets stored.)
Why Zyprexa (And Other Atypical Antipsychotics) Make You Fat
пятница, 15 октября 2010 г.
пролонгированная форма рисперидона vs кветиапин
Chronic management of schizophrenia and schizoaffective disorders is frequently complicated by symptomatic relapse. An open-label, randomized, active-controlled, 2-year trial evaluated 710 patients with schizophrenia or related disorders who were switched from stable treatment with oral risperidone, olanzapine, or conventional neuroleptics to risperidone long-acting injectable (RLAI) or oral quetiapine. Primary effectiveness evaluation was time-to-relapse. Safety evaluations included adverse events (AEs) reported for the duration of the study, Extrapyramidal Symptom Rating Scale (ESRS), clinical laboratory tests, and vital signs. A total of 666 patients (n=329 RLAI, n=337 quetiapine) were evaluable for effectiveness measures. Baseline demographics were similar between treatment groups. Kaplan–Meier estimate of time-to-relapse was significantly longer with RLAI (p < 0.0001). Relapse occurred in 16.5% of patients with RLAI and 31.3% with quetiapine. RLAI and quetiapine were both safe and well tolerated. Weight gain affected 7% of patients with RLAI and 6% with quetiapine, with mean end point increases of 1.25±6.61 and 0±6.55 kg, respectively. There were no significant between-group differences in weight gain. ESRS total scores decreased similarly after randomization to either RLAI or quetiapine. Extrapyramidal AEs occurred in 10% of patients with RLAI and 6% with quetiapine. Treatment-emergent potentially prolactin-related AEs were reported in 15 (5%) patients with RLAI and 5 (2%) patients with quetiapine; hyperprolactinemia was reported in 43 (13.1%) patients with RLAI and 5 (1.5%) patients with quetiapine. Somnolence occurred in 2% of patients with RLAI and 11% with quetiapine. To our knowledge, this is the first report of a randomized clinical trial directly comparing relapse prevention with a second-generation long-acting injectable antipsychotic and oral therapy. Time-to-relapse in stable patients with schizophrenia or schizoaffective disorder was significantly longer in patients randomized to RLAI compared with those randomized to oral quetiapine. Both antipsychotics were generally well tolerated.
Relapse Prevention in Schizophrenia and Schizoaffective Disorder with Risperidone Long-Acting Injectable vs Quetiapine: Results of a Long-Term, Open-Label, Randomized Clinical Trial
среда, 6 октября 2010 г.
Влияние гипертензии и индекса массы тела на когнитивные функции у больных шизофренией
Objective: In recent years there has been a greater appreciation of the elevated prevalence of cardiovascular risk factors in the schizophrenia population and the liability some treatments have for their development. These vascular risk factors are in turn important risk factors in the development of dementia and more subtle cognitive impairments. However, their impact on the cognitive functions of patients with schizophrenia remains underexplored. The authors investigated whether vascular risk factors influence the cognitive impairments of schizophrenia and whether their effects on cognition in schizophrenia are different from those observed in nonpsychiatric comparison subjects.
Method: The authors compared 100 patients with schizophrenia and 53 comparison subjects on cognitive test performance in 2x2 matrices composed of individual vascular risk factors and group (schizophrenia patients and comparison subjects).
Results: Hypertension exerted a significant negative effect on immediate delayed and recognition memory in both groups. Patients with schizophrenia and hypertension were adversely affected in recognition memory, whereas comparison subjects were not. A body mass index above 25 was associated with negative effects on delayed memory in both groups, although the association fell short of statistical significance.
Conclusions: Given that patients with schizophrenia have a higher prevalence of vascular risk factors than the general population and are undertreated for them, treatment of these risk factors may significantly improve cognitive outcome in schizophrenia.
The Effects of Hypertension and Body Mass Index on Cognition in Schizophrenia
среда, 15 сентября 2010 г.
Роль аппетита в прогнозе набора массы тела при терапии оланзапином
Early weight changes may be a more useful predictor for long-term weight changes than early score changes on appetite assessment scales.
The potential role of appetite in predicting weight changes during treatment with olanzapine
четверг, 8 июля 2010 г.
Низкий уровень холестерина как фактор риска аффективных расстройств
In the early 1990s several studies suggested a link between low cholesterol (< 160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Multiple randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent
Cholesterol, mood, and vascular health: Untangling the relationship
вторник, 11 мая 2010 г.
Амантадин для коррекции набора веса при терапии оланзапином
OBJECTIVE: This study sought to determine if amantadine affects weight gain in psychiatric patients taking olanzapine. METHOD: Twenty-one adults who had gained at least 5 lb with olanzapine were randomly assigned to receive amantadine (N=12) or placebo (N=9) in addition to olanzapine. The length of time taking olanzapine ranged from 1 to 44 months. Body mass index, psychiatric status, and fasting blood levels were assessed at baseline and 12 weeks. RESULTS: Significantly fewer subjects taking amantadine gained weight, with a mean change in body mass index of –0.07 kg/m2 for the amantadine group and 1.24 kg/m2 for the placebo group. This effect remained significant when the authors controlled for baseline body mass index and length of olanzapine treatment. No changes in fasting glucose, insulin, leptin, prolactin, and lipid levels were seen. Positive and Negative Syndrome Scale scores remained stable. CONCLUSIONS: Amantadine induced weight stabilization in subjects taking olanzapine and was well tolerated.
Double-Blind, Placebo-Controlled Investigation of Amantadine for Weight Loss in Subjects Who Gained Weight With Olanzapine
Double-Blind, Placebo-Controlled Investigation of Amantadine for Weight Loss in Subjects Who Gained Weight With Olanzapine
четверг, 25 марта 2010 г.
Эффективность метформина и топирамата в снижении набора веса вызванного терапией атипичными антипсихотиками
OBJECTIVE: To review the literature describing the efficacy of metformin and topiramate for the treatment of second-generation antipsychotic–induced weight gain.
DATA SOURCES: Articles were identified by searching the MEDLINE database (from 1949 through January 2010) using the key words metformin, topiramate, antipsychotic, weight, weight gain, and obesity.
STUDY SELECTION AND DATA EXTRACTION: All randomized, placebo-controlled trials of metformin and topiramate were selected for review.
DATA SYNTHESIS: Weight gain due to second-generation antipsychotic use is a concern due to the risk of long-term metabolic and cardiovascular effects with these agents. These effects include obesity, hyperglycemia, and insulin resistance, all of which may contribute to diabetes and cardiovascular disease. Second-generation antipsychotics vary in the degree to which they cause weight gain, and dietary and lifestyle changes may not be feasible or sufficient in counter-acting this weight gain. Although other pharmacologic agents may be beneficial to prevent and treat antipsychotic-induced weight gain, metformin and topiramate have been the most extensively studied in this setting. Metformin acts peripherally to cause weight loss, while topiramate acts centrally. Review of 11 randomized, controlled trials demonstrates beneficial effects of metformin and topiramate in prevention and treatment of weight gain. Metformin is generally well tolerated and has been studied in pediatric patients, while topiramate is associated with more drug interactions and may possibly interfere with control of schizophrenia.
CONCLUSIONS: Data for the use of metformin and topiramate in the treatment and prevention of second-generation antipsychotic–induced weight gain are limited. Both may be effective in helping patients lose weight via mechanisms that have yet to be clearly defined. The use of metformin results in greater weight loss than topiramate, and topiramate is associated with more risks and may compromise the treatment of schizophrenia. Treatment of antipsychotic-induced weight gain with metformin may be an option after lifestyle and dietary changes have failed.
Efficacy of Metformin and Topiramate in Prevention and Treatment of Second-Generation Antipsychotic–Induced Weight Gain
DATA SOURCES: Articles were identified by searching the MEDLINE database (from 1949 through January 2010) using the key words metformin, topiramate, antipsychotic, weight, weight gain, and obesity.
STUDY SELECTION AND DATA EXTRACTION: All randomized, placebo-controlled trials of metformin and topiramate were selected for review.
DATA SYNTHESIS: Weight gain due to second-generation antipsychotic use is a concern due to the risk of long-term metabolic and cardiovascular effects with these agents. These effects include obesity, hyperglycemia, and insulin resistance, all of which may contribute to diabetes and cardiovascular disease. Second-generation antipsychotics vary in the degree to which they cause weight gain, and dietary and lifestyle changes may not be feasible or sufficient in counter-acting this weight gain. Although other pharmacologic agents may be beneficial to prevent and treat antipsychotic-induced weight gain, metformin and topiramate have been the most extensively studied in this setting. Metformin acts peripherally to cause weight loss, while topiramate acts centrally. Review of 11 randomized, controlled trials demonstrates beneficial effects of metformin and topiramate in prevention and treatment of weight gain. Metformin is generally well tolerated and has been studied in pediatric patients, while topiramate is associated with more drug interactions and may possibly interfere with control of schizophrenia.
CONCLUSIONS: Data for the use of metformin and topiramate in the treatment and prevention of second-generation antipsychotic–induced weight gain are limited. Both may be effective in helping patients lose weight via mechanisms that have yet to be clearly defined. The use of metformin results in greater weight loss than topiramate, and topiramate is associated with more risks and may compromise the treatment of schizophrenia. Treatment of antipsychotic-induced weight gain with metformin may be an option after lifestyle and dietary changes have failed.
Efficacy of Metformin and Topiramate in Prevention and Treatment of Second-Generation Antipsychotic–Induced Weight Gain
понедельник, 29 июня 2009 г.
понедельник, 15 июня 2009 г.
Подписаться на:
Сообщения (Atom)